M2512
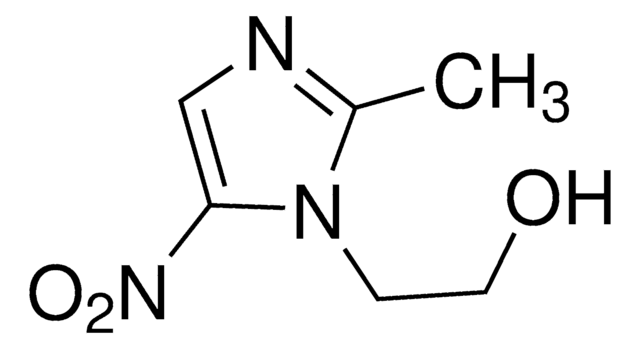
Methyl 2,3-anhydro-4,6-O-benzylidene-α-D-allopyranoside
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H16O5
CAS Number:
Molecular Weight:
264.27
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
storage temp.
2-8°C
Quality Level
SMILES string
COC1OC2COC(OC2C3OC13)c4ccccc4
InChI
1S/C14H16O5/c1-15-14-12-11(18-12)10-9(17-14)7-16-13(19-10)8-5-3-2-4-6-8/h2-6,9-14H,7H2,1H3
InChI key
HQTCRHINASMQOA-UHFFFAOYSA-N
Application
Methyl 2,3-anhydro-4,6-O-benzylidene-α-D-allopyranoside has been used in a study to assess benzylidene acetal ring-opening of a 2-cyano-2-deoxypyranoside derivative. It has also been used in a study to describe new simple routes to the title epoxide using carbonate esters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
María I Mangione et al.
Carbohydrate research, 338(21), 2177-2183 (2003-10-14)
The oxirane ring-opening of an anhydro sugar with diethylaluminum cyanide (Et(2)AlCN) is a direct approach for obtaining a cyano derivative. Methyl 2,3-anhydro-4,6-O-benzylidene-alpha-D-allopyranoside showed anomalous chemical behavior when treated with Et(2)AlCN. The reaction afforded the corresponding beta-cyanohydrin as the minor component
An alternative synthesis of methyl 2,3-anhydro-4,6-O-benzylidene-α-D-allopyranoside using carbonate esters
Raaijmakers, H., et al.
Carbohydrate Research, 238, 185-192 (1993)
Reaction of methyl 2,3-anhydro-4,6-O-benzylidene-α-D-allopyranoside with ethanolamine and 1,4,7,10-tetraoxa-13-azacyclopentadecane
Toth, G., et al.
Carbohydrate Research, 168, 141-145 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service