I100
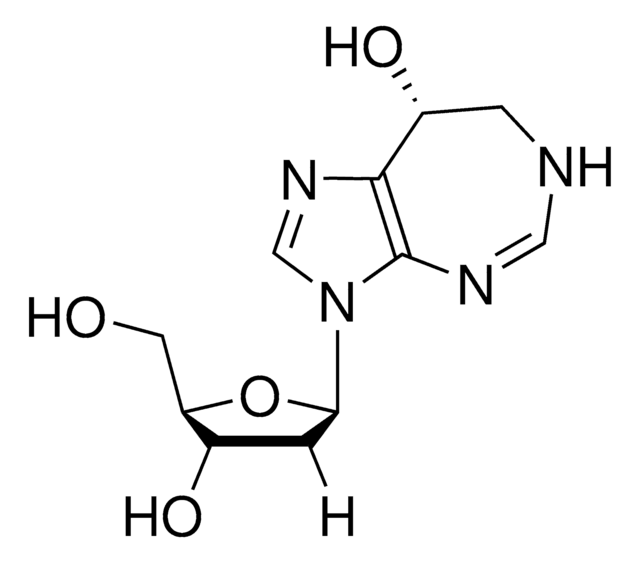
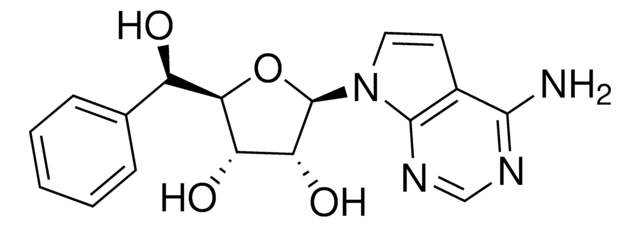
5-Iodotubercidin
≥85%, solid
Synonym(s):
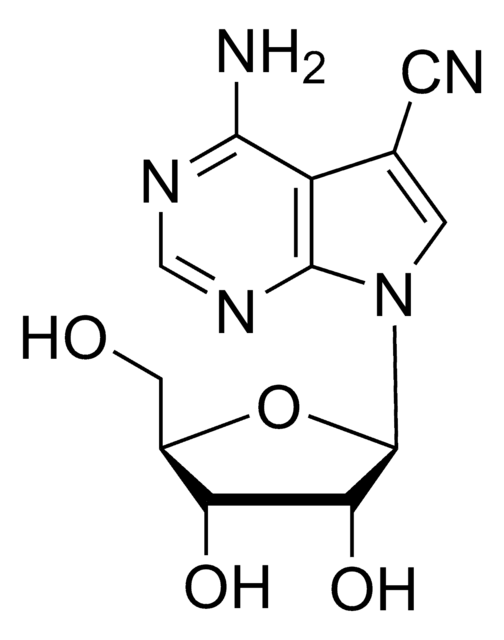
4-Amino-5-iodo-7-(β-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine, 5-Iodotubericidin, 7-Iodo-7-deazaadenosine
About This Item
Recommended Products
Assay
≥85%
form
solid
storage temp.
2-8°C
SMILES string
Nc1ncnc2n(cc(I)c12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7-,8-,11-/m1/s1
InChI key
WHSIXKUPQCKWBY-IOSLPCCCSA-N
Related Categories
General description
Application
Biochem/physiol Actions
Caution
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service