H7630
Hyaluronic acid sodium salt from bovine vitreous humor
Synonym(s):
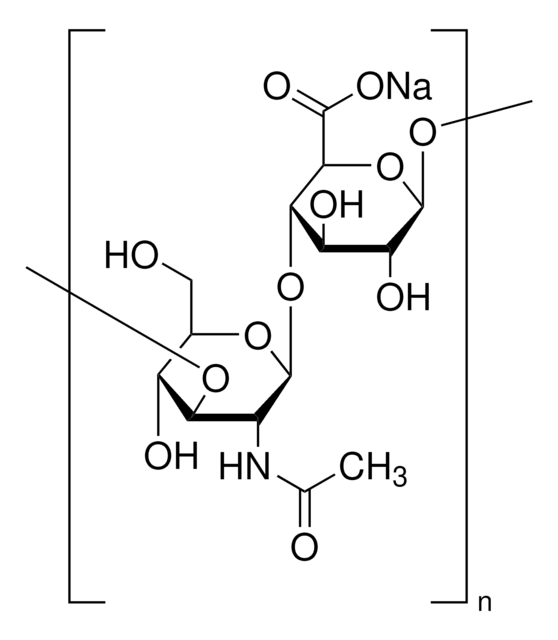
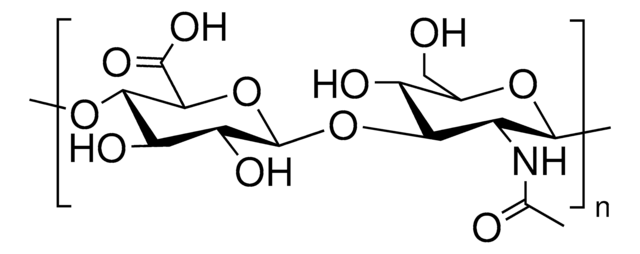
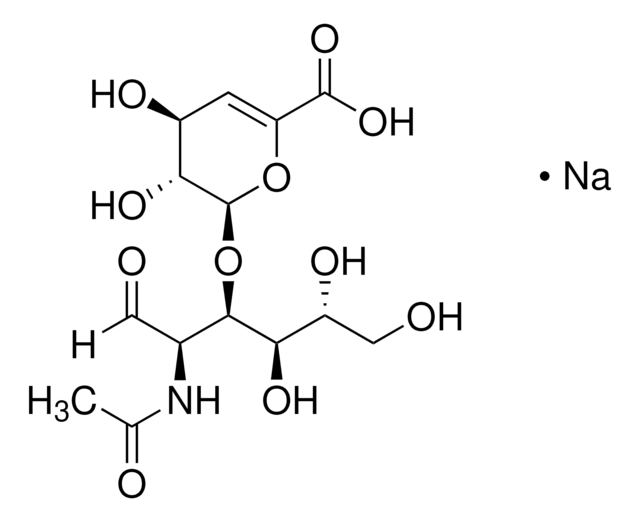
Poly(β-glucuronic acid-[1→3]-β-N-acetylglucosamine-[1→4]), alternating
About This Item
Recommended Products
biological source
bovine (vitreous humor)
Quality Level
form
powder
color
white
solubility
water: ~5 g/L
storage temp.
−20°C
InChI
1S/C28H44N2O23.Na/c1-5(33)29-9-18(11(35)7(3-31)47-25(9)46)49-28-17(41)15(39)20(22(53-28)24(44)45)51-26-10(30-6(2)34)19(12(36)8(4-32)48-26)50-27-16(40)13(37)14(38)21(52-27)23(42)43;/h7-22,25-28,31-32,35-41,46H,3-4H2,1-2H3,(H,29,33)(H,30,34)(H,42,43)(H,44,45);/q;+1/t7-,8-,9-,10-,11-,12-,13+,14+,15-,16-,17-,18-,19-,20+,21+,22+,25-,26+,27-,28-;/m1./s1
InChI key
YWIVKILSMZOHHF-QJZPQSOGSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- as a component for the generation of artificial synovial fluid
- to investigate its role in inducing oxidative stress and interleukin 8(IL-8) production in human nasal RPMI 2650 cells
- to test its effect on B cell proliferation
- as a substrate in neuraminidase assay of morbilliviruses virus
Biochem/physiol Actions
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
There are five identified glycosaminoglycan chains (see Figure 1): Hyaluronan is not sulfated, but the other glycosaminoglycan chains contain sulfate substituents at various positions of the chain.
Glycosaminoglycans are large linear polysaccharides constructed of repeating disaccharide units.
Protocols
This procedure may be used for Hyaluronidase products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service