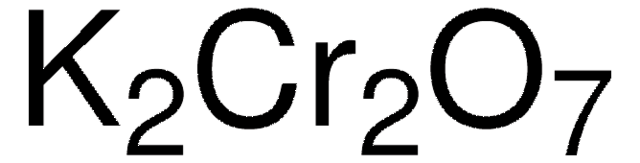71804
Sodium oxalate
reference material for titrimetry, certified by BAM, >99.5%
Synonym(s):
Ethanedioic acid sodium salt, Oxalic acid disodium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NaOOCCOONa
CAS Number:
Molecular Weight:
134.00
Beilstein:
3631622
EC Number:
MDL number:
UNSPSC Code:
41116107
eCl@ss:
39021701
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
reference material
Quality Level
Assay
>99.5%
quality
certified by BAM
technique(s)
titration: suitable
format
neat
SMILES string
[Na+].[Na+].[O-]C(=O)C([O-])=O
InChI
1S/C2H2O4.2Na/c3-1(4)2(5)6;;/h(H,3,4)(H,5,6);;/q;2*+1/p-2
InChI key
ZNCPFRVNHGOPAG-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Sodium oxalate is a volumetric standard produced under stringent conditions and analyzed with the highest possible precision. The content determination and homogeneity assessment of the reductometric standard are carried out using volumetric redox titration as the primary analysis method.
Application
The reference material is used as a reductometric standard for titer determinations in redox titrations.
Features and Benefits
- Available as a solid in a secure glass bottle to ensure its stability for the entire shelf life until opened.
- Traceable to NIST standard reference material
- High-quality offering accurate titer determinations
- Accompanied by a certificate of analysis (CoA)
Analysis Note
Exact concentration, expiry date, traceability and detailed information on certification can be found on the certificate and certification report, included in each package. Content and expiry date on label.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Murthy P.C
University Chemistry, 1 (2008)
Hong-Joo Lee et al.
Bioresource technology, 130, 211-217 (2013-01-12)
The present study investigated the feasibility of the recovery and reuse oxalic acid in a multistage process for the pretreatment of a lignocellulosic biomass. Electrodialysis (ED), an electrochemical process using ion exchange membranes, was used to recover and reuse oxalic
Jiří Jan et al.
Water research, 47(2), 547-557 (2012-12-12)
Correct identification of P forms together with their main Fe and Al binding partners in non-calcareous sediments is of crucial importance for evaluation of P cycling in water bodies. In this paper, we assess extraction methods frequently used for this
Peiyan Li et al.
Food chemistry, 142, 72-78 (2013-09-05)
Effects of oxalic acid on chilling injury, proline metabolism and energy status in mango fruit were investigated after mango fruit (Mangifera indica L. cv. Zill) were dipped in 5mM oxalic acid solution for 10min at 25°C and then stored at
Lars Poulsen et al.
PloS one, 7(12), e50596-e50596 (2012-12-20)
Acid formation in Aspergillus niger is known to be subjected to tight regulation, and the acid production profiles are fine-tuned to respond to the ambient pH. Based on transcriptome data, putative trans-acting pH responding transcription factors were listed and through
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service