850400C
Avanti
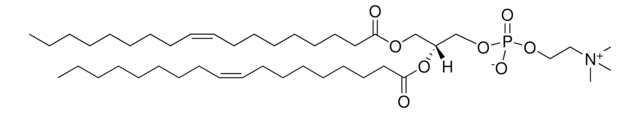
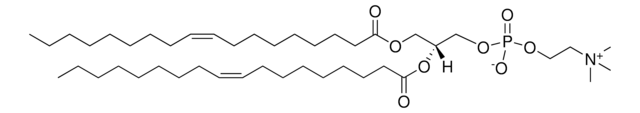
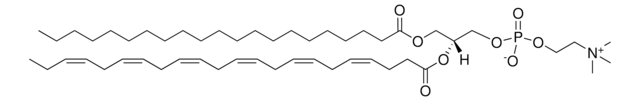
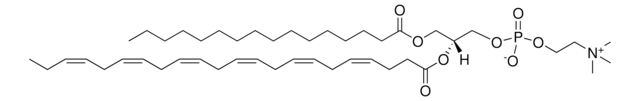
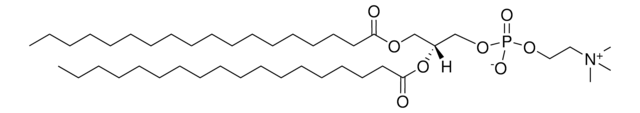
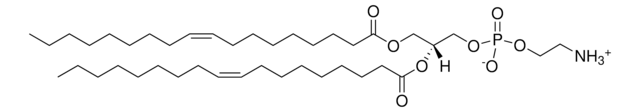
22:6 (Cis) PC
1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine, chloroform
Synonym(s):
1,2-di-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycero-3-phosphocholine; PC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z))
About This Item
Recommended Products
Assay
>99% (TLC)
form
liquid
packaging
pkg of 1 × 2.5 mL (850400C-25mg)
pkg of 5 × 4 mL (850400C-500mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 850400C
concentration
10 mg/mL (850400C-25mg)
25 mg/mL (850400C-500mg)
lipid type
cardiolipins
phospholipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
[P](=O)([O-])(OC[C@H](OC(=O)CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC)COC(=O)CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC)OCC[N+](C)(C)C
InChI
1S/C52H80NO8P/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-36-38-40-42-44-51(54)58-48-50(49-60-62(56,57)59-47-46-53(3,4)5)61-52(55)45-43-41-39-37-35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h8-11,14-17,20-23,26-29,32-35,38-41,50H,6-7,12-13,18-19,24-25,30-31,36-37,42-49H2,1-5H3/b10-8-,11-9-,16-14-,17-15-,22-20-,23-21-,28-26-,29-27-,34-32-,35-33-,40-38-,41-39-/t50-/m1/s1
InChI key
XLKQWAMTMYIQMG-SVUPRYTISA-N
General description
Application
- in liposomes, to study its effect on membrane vesiculation by dynamin and endophilin
- in multi-lamellar vesicles (MLVs) to analyze its effect on the biophysical properties of lipid membranes and on its interaction with a fragment of the Aβ peptide
- in lipid bilayers to study the influence of cholesterol on lateral segregation of saturated and unsaturated phospholipids
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Target Organs
Central nervous system, Liver,Kidney
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flash Point(F)
does not flash
Flash Point(C)
does not flash
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service