T206
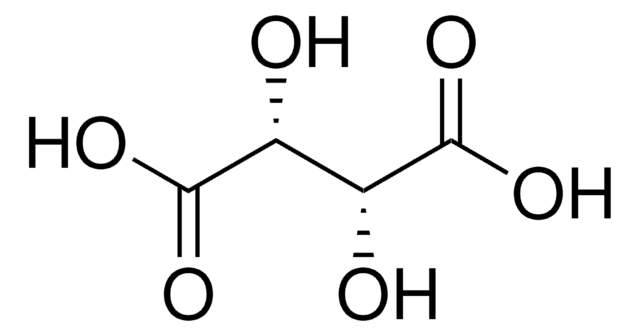
D-(−)-Tartaric acid
ReagentPlus®, 99%
Synonym(s):
(2S,3S)-(−)-Tartaric acid, D-Threaric acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HO2CCH(OH)CH(OH)CO2H
CAS Number:
Molecular Weight:
150.09
Beilstein:
1725145
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
description
synthetic
Quality Level
product line
ReagentPlus®
Assay
99%
optical activity
[α]20/D −12°, c = 20 in H2O
optical purity
ee: 99% (GLC)
mp
172-174 °C (lit.)
solubility
water: soluble 100 mg/mL, clear, colorless
SMILES string
O[C@@H]([C@H](O)C(O)=O)C(O)=O
InChI
1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m0/s1
InChI key
FEWJPZIEWOKRBE-LWMBPPNESA-N
Looking for similar products? Visit Product Comparison Guide
General description
D-(-)-Tartaric acid is a polycrystalline solid, widely used as food additive. It has been reported to exhibit piezoelectric effect.
D-(−)-Tartaric acid is a polyhydroxy acid. Oxidation of d-tartaric acid has been reported. Crystal structure of D-(−)-tartaric acid has been studied by X-ray and neutron diffraction. Tartaric acid is reported to be one of the constituents of soy bean Lipositol. Tartaric acid assists in the generation Y2O3:Eu3+ nanoparticles by sol–gel method. Tartaric acid is the main acid present in grapes and red wine.
Application
D-(-)-Tartaric acid has been used in the near-field, terahertz time-domain spectroscopic (THz-TDS) analysis of waveguides filled with polycrystalline D-tartaric acid, and with polyethylene and silicon powders.
D-(−)-Tartaric acid may be used in the synthesis of the HIV-protease inhibitor nelfinavir. It may be used in the synthesis of chiral aziridine derivative, a common intermediate for the synthesis of hydroxyethylamine class HIV protease inhibitors such as saquinavir, amprenavir, or nelfinavir.
Other Notes
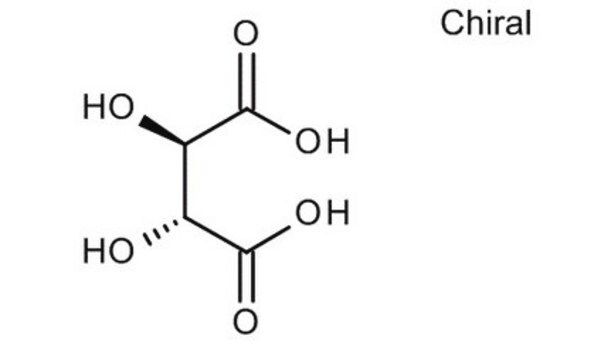
Unnatural isomer
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B M Kim et al.
Organic letters, 3(15), 2349-2351 (2001-07-21)
[reaction: see text] Chiral aziridine derivative 1 was prepared from D-tartaric acid. This compound could be utilized as a common intermediate for the synthesis of hydroxyethylamine class HIV protease inhibitors such as saquinavir, amprenavir, or nelfinavir.
A synthesis of the HIV-protease inhibitor nelfinavir from D-tartaric acid.
Albizati KF, et al.
Tetrahedron Letters, 42(37), 6481-6485 (2001)
M I Gil et al.
Journal of agricultural and food chemistry, 48(10), 4581-4589 (2000-10-29)
The antioxidant activity of pomegranate juices was evaluated by four different methods (ABTS, DPPH, DMPD, and FRAP) and compared to those of red wine and a green tea infusion. Commercial pomegranate juices showed an antioxidant activity (18-20 TEAC) three times
Refinement of the structure of D-tartaric acid by x-ray and neutron diffraction.
Okaya Y, et al.
Acta Crystallographica, 21(2), 237-242 (1966)
Terahertz near-field microspectroscopy.
Knab JR, et al.
Applied Physics Letters, 97(3), 031115-031115 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service