All Photos(1)
About This Item
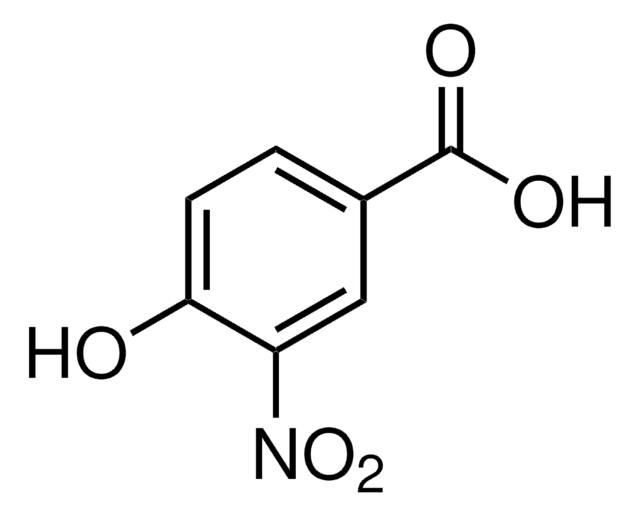
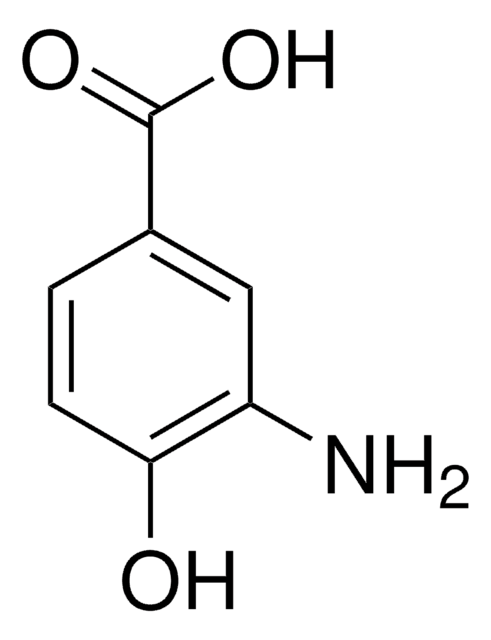
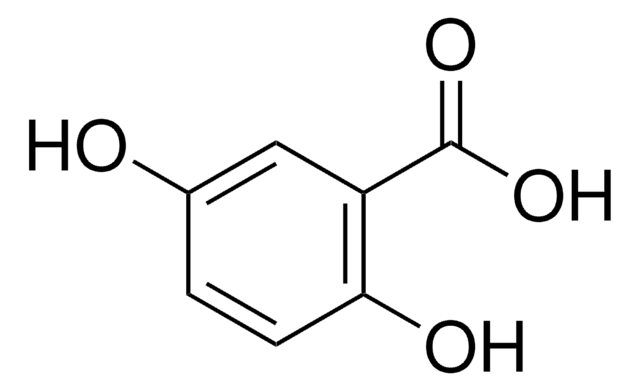
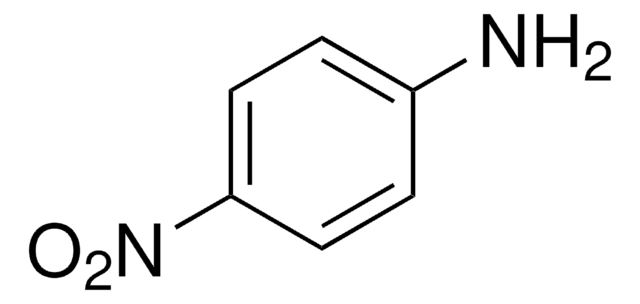
Linear Formula:
HOC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
183.12
Beilstein:
2107564
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
229-231 °C (lit.)
SMILES string
OC(=O)c1ccc(c(O)c1)[N+]([O-])=O
InChI
1S/C7H5NO5/c9-6-3-4(7(10)11)1-2-5(6)8(12)13/h1-3,9H,(H,10,11)
InChI key
XLDLRRGZWIEEHT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Midori Natsume et al.
Journal of clinical biochemistry and nutrition, 42, 50-53 (2008-01-31)
Previously, we identified four metabolites of (-)-epicatechin in blood and urine: (-)-epicatechin-3'-O-glucuronide (E3'G), 4'-O-methyl-(-)-epicatechin-3'-O-glucuronide (4'ME3'G), (-)-epicatechin-7-O-glucuronide (E7G), and 3'-O-methyl-(-)-epicatechin-7-O-glucuronide (3'ME7G) (Natsume et al. Free Radical Biol. Med. 34, 840-849, 2003). The aim of the current study was to compare the
Daiki Asakawa
Journal of the American Society for Mass Spectrometry, 30(8), 1491-1502 (2019-05-31)
Nitrogen-centered and β-carbon-centered hydrogen-deficient peptide radicals are considered to be intermediates in the matrix-assisted laser desorption/ionization in-source decay (MALDI-ISD)-induced Cα-C bond cleavage of peptide backbones when using an oxidizing matrix. To understand the general mechanism of Cα-C bond cleavage by
Yuko Fukuyama et al.
Journal of the American Society for Mass Spectrometry, 29(11), 2227-2236 (2018-08-01)
In in-source decay (ISD) in matrix-assisted laser desorption/ionization (MALDI)-mass spectrometry (MS), 1,5-diaminonaphthalene (1,5-DAN) is a most frequently used matrix probably due to the highly sensitive detection of fragment ions. 1,5-DAN is a reducing matrix generating c- and z-series ions by
Cosima Damiana Calvano et al.
Analytical and bioanalytical chemistry, 410(17), 4015-4038 (2018-04-24)
Since its introduction in the 1980s, matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) has gained a prominent role in the analysis of high molecular weight biomolecules such as proteins, peptides, oligonucleotides, and polysaccharides. Its application to low molecular weight compounds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service