726656
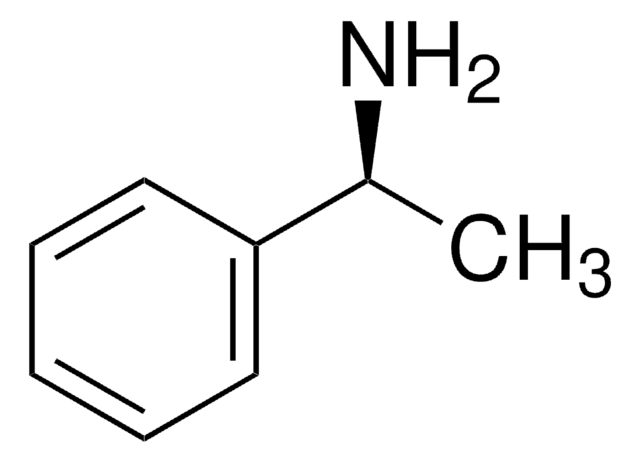
(S)-(−)-4-Methoxy-α-methylbenzylamine
ChiPros®, produced by BASF, 99%
Synonym(s):
(S)-(−)-1-(4-Methoxyphenyl)ethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H13NO
CAS Number:
Molecular Weight:
151.21
Beilstein:
3196456
MDL number:
UNSPSC Code:
12352112
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
produced by BASF
Quality Level
Assay
≥98.5% (GC)
99%
form
liquid
optical purity
enantiomeric excess: ≥98.5%
density
1.024 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
COc1ccc(cc1)[C@H](C)N
InChI
1S/C9H13NO/c1-7(10)8-3-5-9(11-2)6-4-8/h3-7H,10H2,1-2H3/t7-/m0/s1
InChI key
JTDGKQNNPKXKII-ZETCQYMHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(S)-(−)-4-Methoxy-α-methylbenzylamine is employed in the synthesis of S(+)-4-(1-phenylethylamino)quinazolines, as human immunoglobuline E inhibitor and haloaryl-β-amino acids. It is also used as a precursor to prepare chiral intermediate in the total synthesis of solanoeclepin A.
Legal Information
ChiPros is a registered trademark of BASF SE
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S (+)-4-(1-phenylethylamino) quinazolines as inhibitors of human immunoglobuline E synthesis: potency is dictated by stereochemistry and atomic point charges at N-1.
Berger M, et al.
Journal of Medicinal Chemistry, 44(18), 3031-3038 (2001)
The asymmetric synthesis of β-haloaryl-β-amino acid derivatives.
Bull S D, et al.
Synlett, 2000(09), 1257-1260 (2000)
Novel Synthesis of the ABC Rings of Solanoeclepin A.
Lin Y T, et al.
Organic Letters, 16(22), 5948-5951 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service