710733
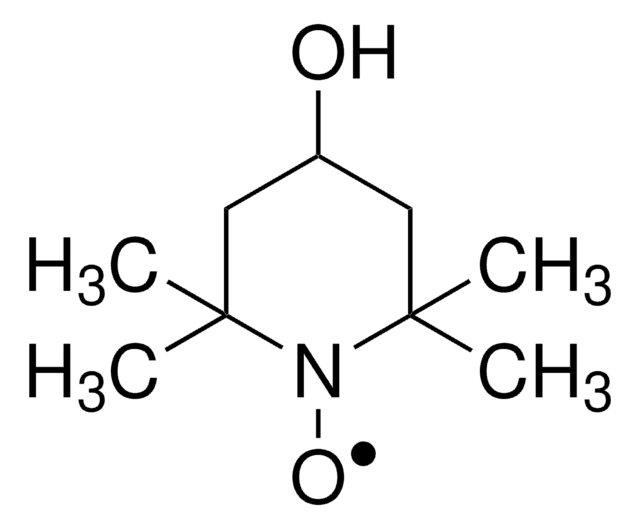
2,2,5-Trimethyl-4-phenyl-3-azahexane-3-nitroxide
Synonym(s):
1,1-Dimethylethyl-2-methyl-1-phenylpropyl nitroxide, tert-Butyl 1-phenyl-2-methylpropyl nitroxide, NMP universal alkoxyamine nitroxide
About This Item
Recommended Products
refractive index
n20/D 1.515
density
0.953 g/mL at 25 °C
storage temp.
−20°C
SMILES string
CC(C)C(N([O])C(C)(C)C)c1ccccc1
InChI
1S/C14H22NO/c1-11(2)13(15(16)14(3,4)5)12-9-7-6-8-10-12/h6-11,13H,1-5H3
InChI key
VGHCMXLEZFMZOZ-UHFFFAOYSA-N
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Ox. Liq. 3
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
205.0 °F - closed cup
Flash Point(C)
96.1 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
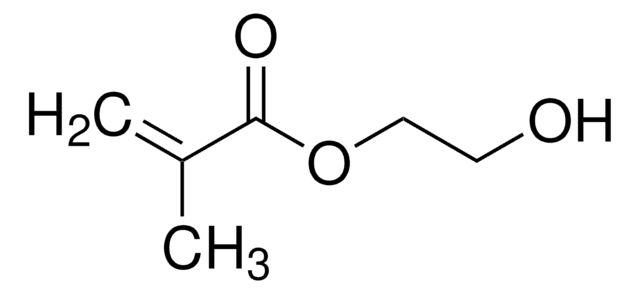
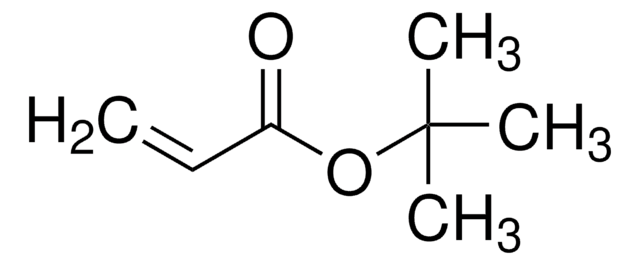
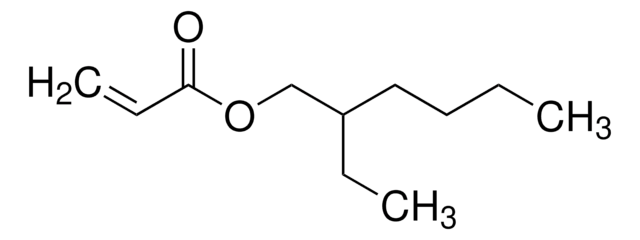
Customers Also Viewed
Articles
A detailed article on block copolymer synthesis using a nitroxide-mediated radical polymerization (NMP) approach.
Block copolymer synthesis using a commercially available nitroxide-mediated radical polymerization (NMP) initiator
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Tools for Performing ATRP
Protocols
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Sigma-Aldrich presents an article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service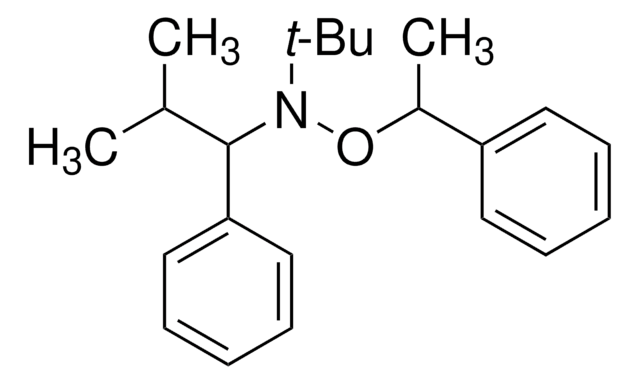
![N-tert-Butyl-O-[1-[4-(chloromethyl)phenyl]ethyl]-N-(2-methyl-1-phenylpropyl)hydroxylamine](/deepweb/assets/sigmaaldrich/product/structures/298/481/e5f98578-0ed3-446e-bed2-e277dfa06e5d/640/e5f98578-0ed3-446e-bed2-e277dfa06e5d.png)