All Photos(1)
About This Item
Empirical Formula (Hill Notation):
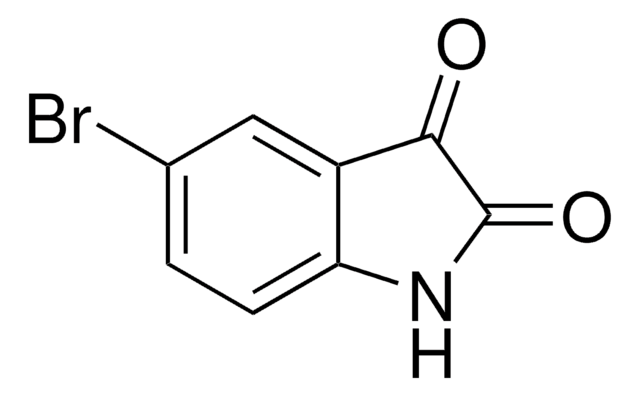
C9H7NO2
CAS Number:
Molecular Weight:
161.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
79-82 °C (lit.)
functional group
ester
SMILES string
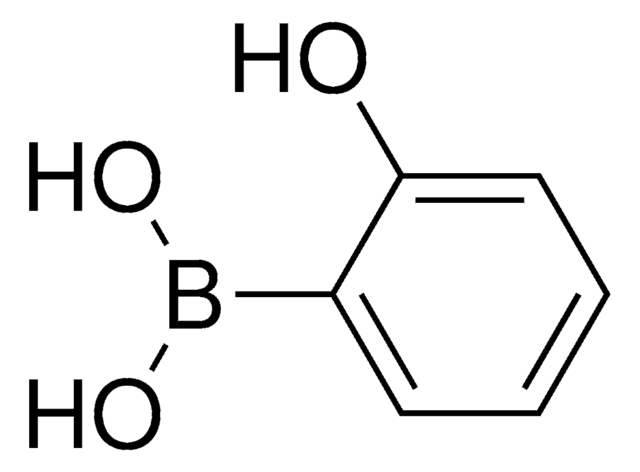
CC1=Nc2ccccc2C(=O)O1
InChI
1S/C9H7NO2/c1-6-10-8-5-3-2-4-7(8)9(11)12-6/h2-5H,1H3
InChI key
WMQSKECCMQRJRX-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B Siedle et al.
Bioorganic & medicinal chemistry, 10(9), 2855-2861 (2002-07-12)
Human neutrophil elastase (HNE) is a serine protease that has been implicated in the abnormal turnover of connective tissue proteins and has been described as an important pathogenic factor in several inflammatory diseases such as rheumatoid arthritis or cystic fibrosis.
Reactivity of 4H-3,1-benzoxazin-4-ones towards nitrogen and carbon nucleophilic reagents: applications to the synthesis of new heterocycles.
Madkour HMF.
ARKIVOC (Gainesville, FL, United States), 1, 36-54 (2004)
A Simple Synthesis of 2-Substituted-4 H-3, 1-benzoxazin-4-ones by Palladium-Catalyzed Cyclocarbonylation of o-Iodoanilines with Acid Chlorides.
Chitchamai L and Alper H.
Organic Letters, 1(10), 1619-1622 (1999)
L Hedstrom et al.
Biochemistry, 23(8), 1753-1759 (1984-04-10)
The benzoxazinones 2-ethoxy-4H-3,1- benzoxazin -4-one (1a) and 2-(trifluoromethyl)-4H-3,1- benzoxazin -4-one (1d) inactivate chymotrypsin. The inactivation is stoichiometric and proceeds with rate constants of 7 X 10(5) M-1 min-1 and greater than 4 X 10(6) M-1 min-1, respectively. The inactivated enzyme
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![9,10-Difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid 97%](/deepweb/assets/sigmaaldrich/product/structures/226/667/f62b7b1a-a8c8-42cf-ba23-84a9408985be/640/f62b7b1a-a8c8-42cf-ba23-84a9408985be.png)