40407
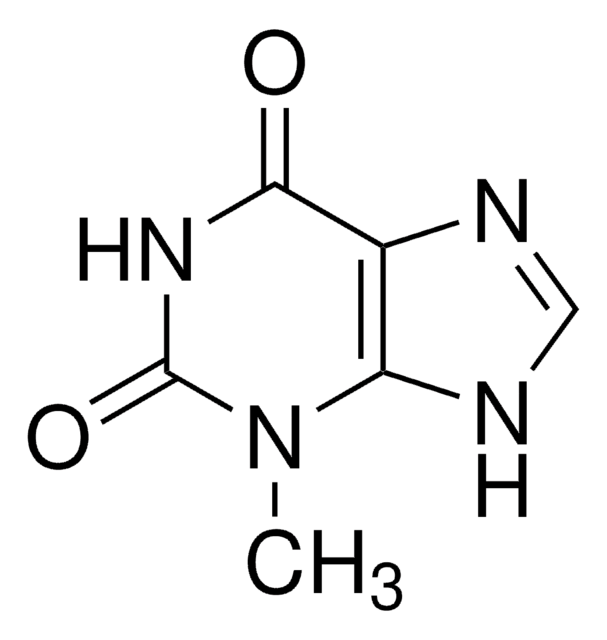
1,7-Dimethyluric acid
≥97.0% (HPLC)
Synonym(s):
1,7-Dimethyl-2,6,8-trihydroxypurine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H8N4O3
CAS Number:
Molecular Weight:
196.16
Beilstein:
219682
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (HPLC)
SMILES string
CN1C(=O)NC2=C(N(C)C(=O)N2)C1=O
InChI
1S/C7H8N4O3/c1-10-3-4(8-6(10)13)9-7(14)11(2)5(3)12/h1-2H3,(H,8,13)(H,9,14)
InChI key
NOFNCLGCUJJPKU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,7-Dimethyluric acid is an important metabolite of caffeine. Electrochemical oxidation of 1,7-dimethyluric acid was studied over a wide pH range of 2.2-10.3 at solid electrodes.
Application
1,7-Dimethyluric acid is the suitable reagent used for the simultaneous determination of plasma levels of theophylline and its metabolites without interference from caffeine or caffeine metabolites by HPLC.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Electrochemical and peroxidase catalysed oxidation of 1, 7-dimethyluric acid and effect of methyl groups on the oxidation mechanism.
Goyal RN, et al.
J. Chem. Soc. Perkin Trans. II, 6, 1153-1159 (1996)
J Kizu et al.
Biomedical chromatography : BMC, 13(1), 15-23 (1999-04-07)
A high performance liquid chromatography (HPLC) method has been developed for the simultaneous determination of plasma levels of theophylline and its metabolites without interference from caffeine or caffeine metabolites. The method is simple and of practical use because it is
M T Landi et al.
Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 5(9), 693-698 (1996-09-01)
Cytochrome P4501A2 (CYP1A2) activity may be related to bladder cancer risk through metabolic activation of aromatic amines, such as 4-aminobiphenyl (ABP), to reactive intermediates that can form DNA and hemoglobin (Hb) adducts. In the context of a study on smoking
M E Campbell et al.
Clinical pharmacology and therapeutics, 42(2), 157-165 (1987-08-01)
Systemic caffeine clearance and urinary metabolite profiles were determined in 15 subjects with diverse exposure histories to cytochrome P-450 inducers (cigarette smoke) and inhibitors (oral contraceptive steroids). A correlation was observed between caffeine clearance and a urinary ratio based on
E Asprodini et al.
The Journal of pharmacology and experimental therapeutics, 368(2), 262-271 (2018-12-29)
The purpose of the study was to determine whether the in vivo activities of drug-metabolizing enzymes CYP1A2 and CYP2A6, xanthine oxidase (XO), and N-acetyltransferase-2 (NAT2) vary across the menstrual cycle. Forty-two healthy women were studied at early follicular phase (EFP:
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service