32851
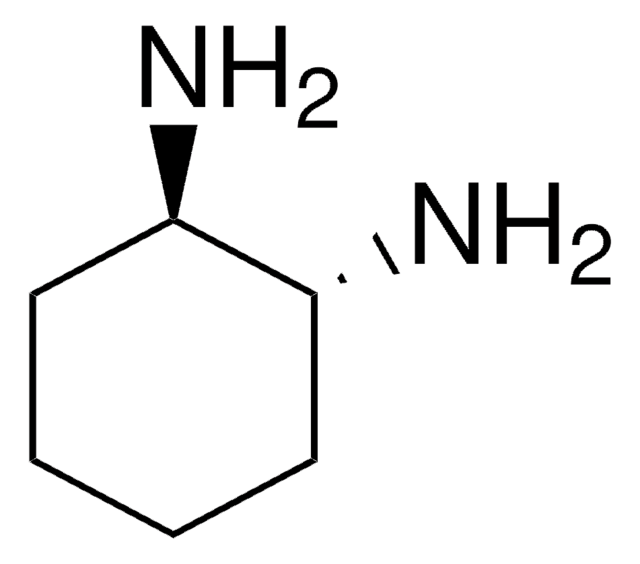
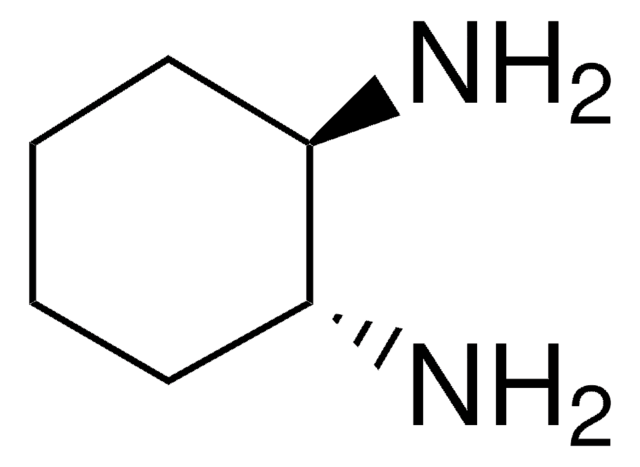
trans-1,4-Diaminocyclohexane
≥98.0% (GC)
Synonym(s):
trans-1,4-Cyclohexanediamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H10(NH2)2
CAS Number:
Molecular Weight:
114.19
Beilstein:
2801657
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
18 mmHg ( 87.2 °C)
Assay
≥98.0% (GC)
autoignition temp.
680 °F
expl. lim.
6 %
bp
197 °C (lit.)
mp
68-72 °C (lit.)
SMILES string
N[C@H]1CC[C@H](N)CC1
InChI
1S/C6H14N2/c7-5-1-2-6(8)4-3-5/h5-6H,1-4,7-8H2/t5-,6-
InChI key
VKIRRGRTJUUZHS-IZLXSQMJSA-N
Looking for similar products? Visit Product Comparison Guide
Application
trans-1,4-Diaminocyclohexane was used in preparation of fully aliphatic polyimides. It was employed as the structure-directing agent in the synthesis of novel two-dimensional layered zinc phosphate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
159.8 °F - closed cup
Flash Point(C)
71 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hwan-Chul Yu et al.
Journal of nanoscience and nanotechnology, 11(7), 6141-6147 (2011-11-30)
Fully aliphatic polyimides (APIs) were prepared from rel-(1'R,3S5'S)-spiro[furan-3(2H),6'-[3]oxabicyclo[3.2.1]octane]-2,2',4',5(4H)-tetrone (DAn) as unsymmetrical spiro dianhydride, and either cis-trans-1,4-diaminocyclohexane (mix-DACH) or trans-1,4-diaminocyclohexane (trans-DACH) as diamine. Structure of all prepared monomers and polymers was confirmed via 1H-NMR and FT-IR. The solubility, optical transparency, and
Jana Kasparkova et al.
Biochemical pharmacology, 79(4), 552-564 (2009-09-29)
Earlier studies have described promising antitumor activity of a large-ring chelate complex [PtCl(2)(cis-1,4-DACH)] (DACH=diaminocyclohexane). Encouraging antitumor activity of this analogue of cisplatin prompted us to perform studies focused on the mechanistic basis of pharmacological effects of this complex. Four early
J D Hoeschele et al.
Journal of medicinal chemistry, 37(17), 2630-2636 (1994-08-19)
The first two analogs 5a,b of a new class of neutral large-ring square-planar Pt(II) chelate complexes of the generic structure [Pt(cis-1,4-dach)X2] were synthesized via a refined technique, structurally characterized by NMR (1H, 13C, 195Pt), FAB mass spectrometry, and X-ray crystallography
Synthesis and structure of a new layered zinc phosphate [C 6 N 2 H 1 6] 3. 5 [Zn 1 4 (PO 4) 7 (HPO 4) 7] in the presence of trans-1, 4-diaminocyclohexane.
Xing Y, et al.
Inorganic Chemistry Communications, 7(4), 475-477 (2004)
F Vianello et al.
Protein expression and purification, 15(2), 196-201 (1999-03-02)
A novel, simple, and rapid procedure for the purification of pea seedling amine oxidase is reported. The crude enzyme, obtained by ammonium sulfate fractionation, was purified in two steps: the first one by anion-exchange chromatography and the second one by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service