294632
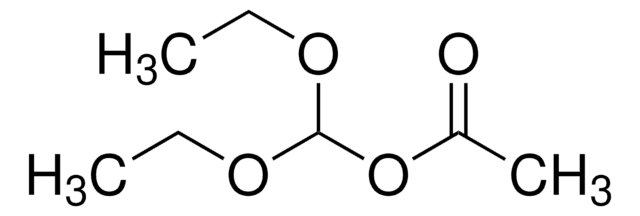
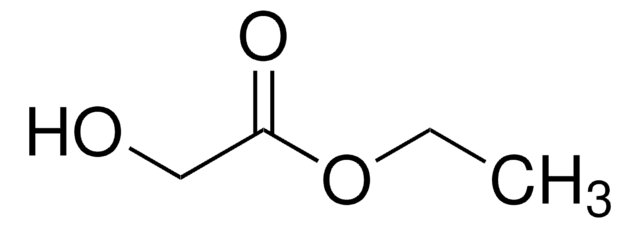
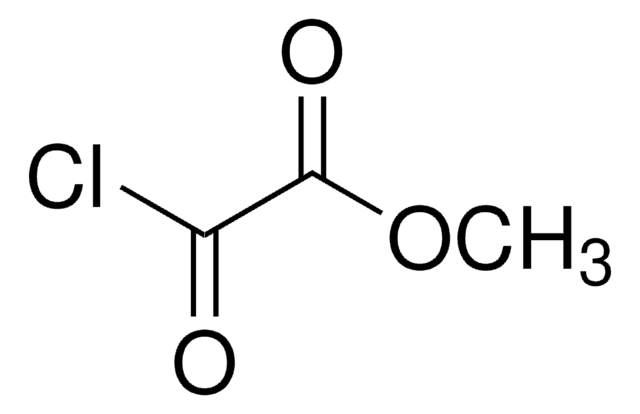
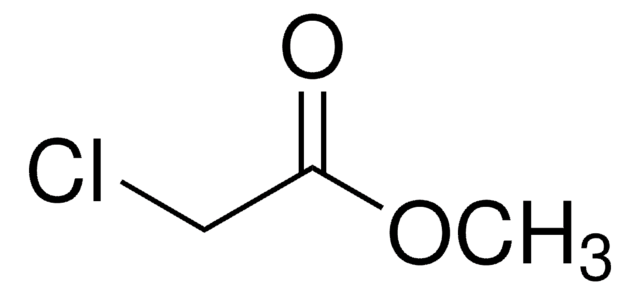
Methyl dimethoxyacetate
97%
Synonym(s):
Glyoxylic acid methyl ester dimethyl acetal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3O)2CHCOOCH3
CAS Number:
Molecular Weight:
134.13
Beilstein:
1757582
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.405 (lit.)
bp
67 °C/18 mmHg (lit.)
density
1.096 g/mL at 25 °C (lit.)
functional group
acetal
ester
ether
storage temp.
2-8°C
SMILES string
COC(OC)C(=O)OC
InChI
1S/C5H10O4/c1-7-4(6)5(8-2)9-3/h5H,1-3H3
InChI key
NZTCVGHPDWAALP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl dimethoxyacetate has been used:
- in Claisen acylation of active hydrogen compounds
- in preparation of 3, 9-disubstituted 2,4,8,10-tetroxaspiro [5.5] undecane
- as Lithium enolate precursor
- as acylating reagent for cycloalkanone enolates and amino alcohols
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Studies in Mixed Ester Condensations. IV. Acylations with Methyl Dimethoxyacetate1.
Royals EE and Robinson AG.
Journal of the American Chemical Society, 78(16), 4161-4164 (1956)
Tetrahedron, 46, 6799-6799 (1990)
Some 3, 9-Dicarboxylic Acids of 2, 4, 8, 10-Tetroxaspiro [5.5] undecane.
CLEMENTS JB and RICE LM.
The Journal of Organic Chemistry, 24(12), 1958-1961 (1959)
P J Connolly et al.
Journal of medicinal chemistry, 36(23), 3674-3685 (1993-11-12)
Compounds comprising a series of 7-[2-(4-fluorophenyl)-4,5,6,7-tetrahydro-2H-indazol-3-yl]-3,5- dihydroxy-6-heptenoic acid sodium salts (18) were synthesized and tested for their ability to inhibit HMG-CoA reductase in a partially purified enzyme preparation and cholesterol biosynthesis from acetate in cultured HEP-G2 cells. Changing the size
Synthetic Communications, 23, 1003-1003 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service