275352
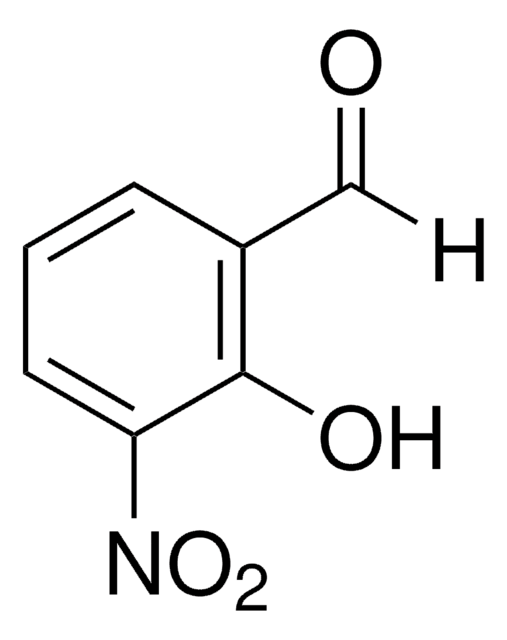
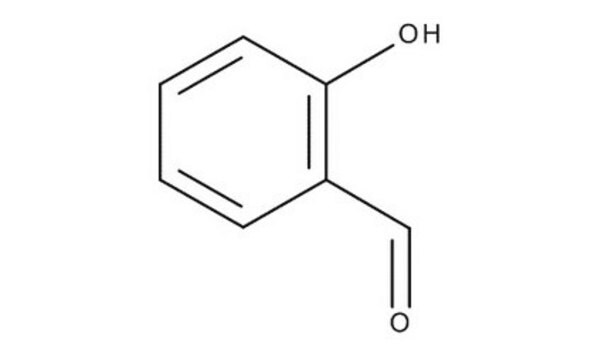
2-Hydroxy-5-nitrobenzaldehyde
98%
Synonym(s):
5-Nitrosalicylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(NO2)CHO
CAS Number:
Molecular Weight:
167.12
Beilstein:
512565
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
125-128 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1cc(ccc1O)[N+]([O-])=O
InChI
1S/C7H5NO4/c9-4-5-3-6(8(11)12)1-2-7(5)10/h1-4,10H
InChI key
IHFRMUGEILMHNU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Hydroxy-5-nitrobenzaldehyde is a nitroaromatic compound used to prepare Schiff base ligands.
The interaction of 2-hydroxy-5-nitrobenzaldehyde and chlorogenic acid (CHL) with the components of the rat hepatic glucose 6-phosphatase system was studied.
The interaction of 2-hydroxy-5-nitrobenzaldehyde and chlorogenic acid (CHL) with the components of the rat hepatic glucose 6-phosphatase system was studied.
Physical properties
Free of 3-nitro isomer
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anke Rüttger et al.
BioTechniques, 41(4), 469-473 (2006-10-31)
A method is described allowing the selective determination of four cathepsins (B, H, K, and L) in live cells. Adherently growing cells are incubated with partially selective substrates for each cathepsin (peptidic derivatives of 4-methoxy-beta-naphthylamine) in microtiter plates together with
W J Arion et al.
Archives of biochemistry and biophysics, 339(2), 315-322 (1997-03-15)
We have studied the interactions of chlorogenic acid (CHL) and 2-hydroxy-5-nitrobenzaldehyde (HNB) with the components of the rat hepatic glucose 6-phosphatase (Glc-6-Pase) system. CHL and HNB are competitive inhibitors of glucose 6-phosphate (Glc-6-P) hydrolysis in intact microsomes with Ki values
M A Pajares et al.
The Journal of biological chemistry, 264(12), 6804-6809 (1989-04-25)
The 11-cis-retinal binding site of rhodopsin is of great interest because it is buried in the membrane but yet must provide an environment for charged amino acids. In addition, the active-site lysine residue must be able to engage in rapid
Anda-Mihaela Olaru et al.
Carbohydrate polymers, 179, 59-70 (2017-11-08)
A series of hydrogels based on chitosan polyamine and nitrosalicylaldehyde were prepared via dynamic covalent chemistry (DCC), by imination and transimination reactions towards ordered clusters which play the role of crosslinking nodes of the chitosan network. The hydrogelation mechanism has
Anas G Elsafy et al.
Sensors (Basel, Switzerland), 18(7) (2018-07-13)
(E)-2-((benzo[d]thiazol-2-ylimino)methyl)-4-nitrophenol 1 and (E)-2-(((6-methoxybenzo[d]thiazol-2-yl)imino)methyl)-4-nitrophenol 2 were synthesized efficiently under microwave conditions. The structures were confirmed using IR, ¹H NMR, and 13C NMR. UV-vis. Fluorescence investigations demonstrated that 1 and 2 are sensitive and selective sensors for detection of cyanide over
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service