27470
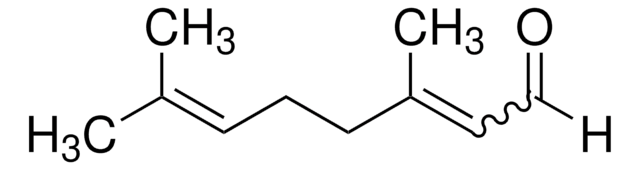
(±)-Citronellal
≥95.0% (GC)
Synonym(s):
(±)-3,7-Dimethyl-6-octenal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
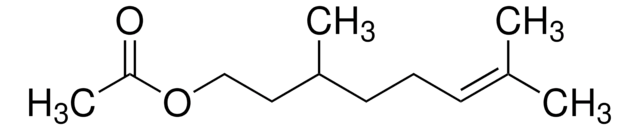
(CH3)2C=CHCH2CH2CH(CH3)CH2CHO
CAS Number:
Molecular Weight:
154.25
Beilstein:
1720789
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0% (GC)
form
liquid
refractive index
n20/D 1.451 (lit.)
bp
207 °C (lit.)
density
0.857 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
CC(CC\C=C(\C)C)CC=O
InChI
1S/C10H18O/c1-9(2)5-4-6-10(3)7-8-11/h5,8,10H,4,6-7H2,1-3H3
InChI key
NEHNMFOYXAPHSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(±)-Citronellal was used to inhibit the development of mechanical nociception induced by carrageenan (CG) and tumor necrosis factor-α (TNF-α).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hajo Kries et al.
The Journal of biological chemistry, 292(35), 14659-14667 (2017-07-14)
The natural product class of iridoids, found in various species of flowering plants, harbors astonishing chemical complexity. The discovery of iridoid biosynthetic genes in the medicinal plant Catharanthus roseus has provided insight into the biosynthetic origins of this class of
Raimundo Wagner de S Aguiar et al.
TheScientificWorldJournal, 2014, 492138-492138 (2014-03-07)
Corymbia citriodora and Cymbopogon nardus essential oils samples were analyzed by GC and GC-MS and their qualitative and quantitative compositions established. The main component of essential oils of C. citriodora and C. nardus was citronellal, at 61.78% and 36.6%, respectively.
Walter S Leal et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18704-18709 (2013-10-30)
The southern house mosquito, Culex quinquefasciatus, has one of the most acute and eclectic olfactory systems of all mosquito species hitherto studied. Here, we used Illumina sequencing to identify olfactory genes expressed predominantly in antenna, mosquito's main olfactory organ. Less
Lucindo José Quintans-Júnior et al.
Journal of orofacial pain, 24(3), 305-312 (2010-07-29)
To evaluate the antinociceptive effects of citronellal (CTL) on formalin-, capsaicin-, and glutamate-induced orofacial nociception in mice and to investigate whether such effects might involve a change in neural excitability. Male mice were pretreated with CTL (50, 100, and 200
Ying-Fang Ting et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(23), 7030-7038 (2010-05-11)
Practical and convenient synthetic routes have been developed for the synthesis of a new class of pyrrolidinyl-camphor derivatives (7 a-h). These novel compounds were screened as catalysts for the direct Michael addition of symmetrical alpha,alpha-disubstituted aldehydes to beta-nitroalkenes. When this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service