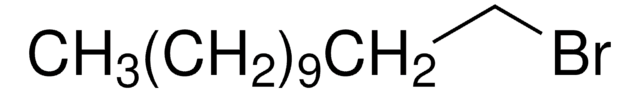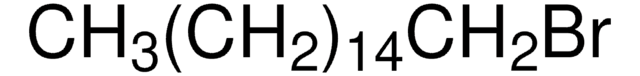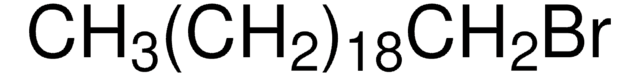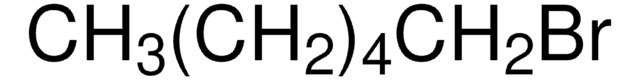199494
1-Bromooctadecane
≥97.0%
Synonym(s):
Octadecyl bromide, Stearyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3(CH2)17Br
CAS Number:
Molecular Weight:
333.39
Beilstein:
774145
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0%
form
solid
bp
214-216 °C/12 mmHg (lit.)
mp
25-30 °C (lit.)
density
0.976 g/mL at 25 °C (lit.)
functional group
alkyl halide
SMILES string
CCCCCCCCCCCCCCCCCCBr
InChI
1S/C18H37Br/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19/h2-18H2,1H3
InChI key
WSULSMOGMLRGKU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Bromooctadecane is a key building block that can undergo nucleophilic substitution. Used to functionalize carbon nanotubes.
Application
1-Bromooctadecane was used in the synthesis of shortened single-walled carbon nanotubes (s-SWCNTs).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kim WJ, et al.
Bull. Korean Chem. Soc., 25(9), 1301-1302 (2004)
Md Shahruzzaman et al.
Journal of separation science, 38(14), 2403-2413 (2015-05-07)
The amphiphilic polymer-grafted silica was newly prepared as a stationary phase in high-performance liquid chromatography. Poly(4-vinylpyridine) with a trimethoxysilyl group at one end was grafted onto porous silica particles and the pyridyl side chains were quaternized with 1-bromooctadecane. The obtained
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service