170275
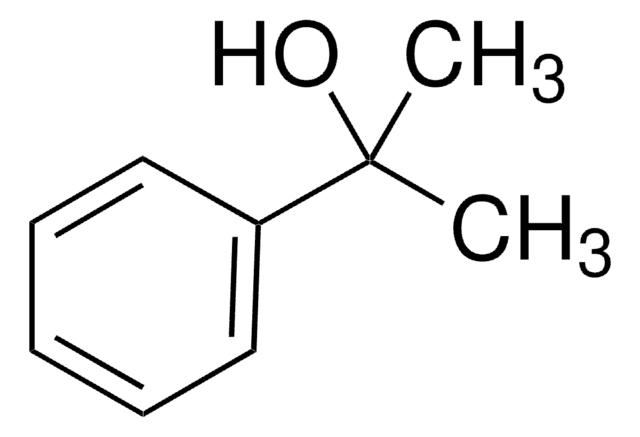
2-Methyl-1-phenyl-2-propanol
98%
Synonym(s):
1,1-Dimethyl-2-phenylethyl alcohol, Benzyl dimethyl carbinol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2C(CH3)2OH
CAS Number:
Molecular Weight:
150.22
Beilstein:
1855608
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
refractive index
n20/D 1.514 (lit.)
bp
94-96 °C/10 mmHg (lit.)
mp
23-25 °C (lit.)
density
0.974 g/mL at 25 °C (lit.)
SMILES string
CC(C)(O)Cc1ccccc1
InChI
1S/C10H14O/c1-10(2,11)8-9-6-4-3-5-7-9/h3-7,11H,8H2,1-2H3
InChI key
RIWRBSMFKVOJMN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Methyl-1-phenyl-2-propanol was used in the preparation of 2-methyl-1-phenyl-2-propyl bromide.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
David C Magri et al.
Organic & biomolecular chemistry, 1(19), 3418-3429 (2003-10-31)
Two dialkyl peroxides, devised as kinetic probes for the heterogeneous electron transfer (ET), are studied using heterogeneous and homogeneous electrochemical techniques. The peroxides react by concerted dissociative ET reduction of the O-O bond. Under heterogeneous conditions, the only products isolated
Takao Raku et al.
Biotechnology letters, 26(8), 665-670 (2004-06-18)
For the purpose of developing a new synthetic polymer containing an asymmetric molecule branch, three racemic alcohols, i.e. 1-phenylethanol, 1-(4-methylphenyl)ethanol and 1-(2-naphthyl)ethanol, were esterified enzymatically with divinyladipate using a lipase from Pseudomonas cepacia. The enzymatic acylation of alcohols produced monoacylated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service