146250
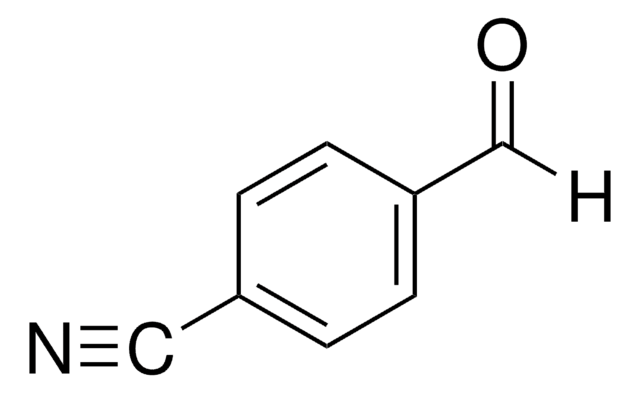
3-Formylbenzonitrile
98%
Synonym(s):
3-Cyanobenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NCC6H4CHO
CAS Number:
Molecular Weight:
131.13
Beilstein:
2205751
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
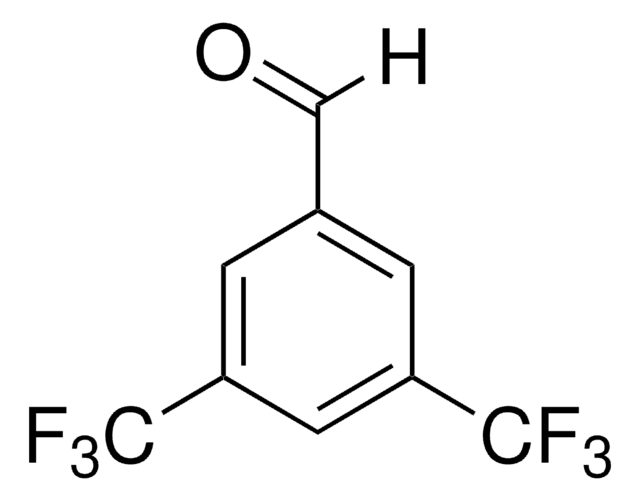
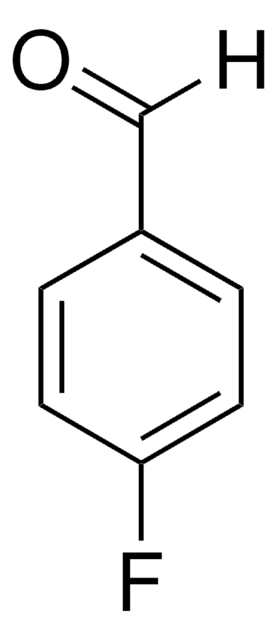
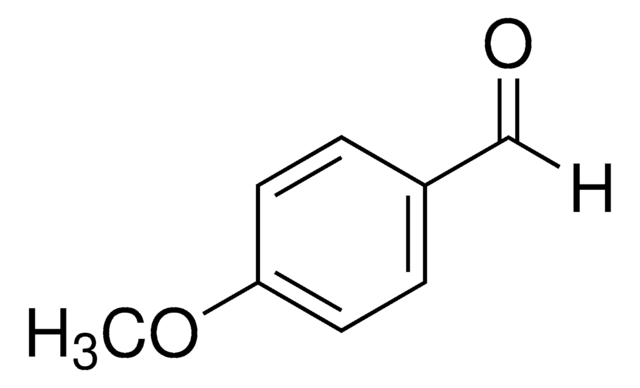
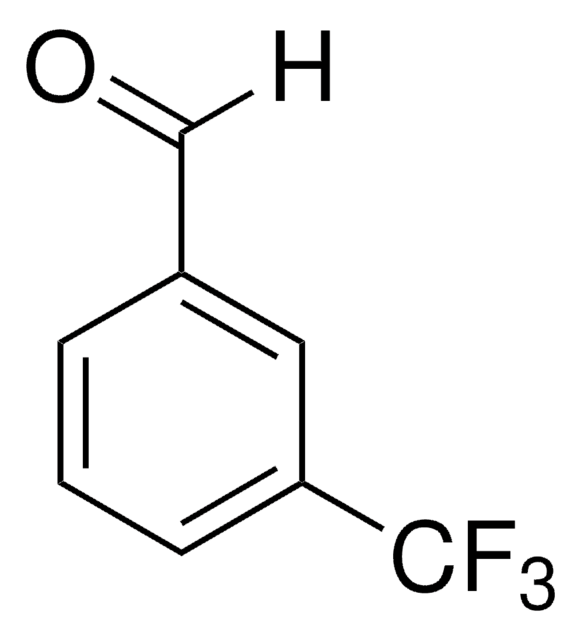
Recommended Products
Assay
98%
form
powder
bp
210 °C (lit.)
mp
75-78 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1cccc(c1)C#N
InChI
1S/C8H5NO/c9-5-7-2-1-3-8(4-7)6-10/h1-4,6H
InChI key
HGZJJKZPPMFIBU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Formylbenzonitrile was used in the synthesis of 3-(6,6-dimethyl-5,6-dihydro-4H-benzo[7,8]chromeno[6,5-d]oxazol-2-yl)benzonitrile.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hong Zhao et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 10(10), 2386-2390 (2004-05-18)
The molten reaction of 2-naphthol, 4-(aminomethyl)pyridine, and 4-pyridinecarboxaldehyde at about 180 degrees C yields trans-2,3-dihydro-2,3-di(4'-pyridyl)benzo[e]indole (1) which possesses two chiral centers, rather than an expected Betti-type reaction product with only one chiral carbon center. The same reactions, using 3-pyridinecarboxaldehyde, 4-cyanobenzaldehyde
Kelly C G Moura et al.
Bioorganic & medicinal chemistry, 20(21), 6482-6488 (2012-09-25)
Twenty-three naphthoimidazoles and six naphthoxazoles were synthesised and evaluated against susceptible and rifampicin- and isoniazid-resistant strains of Mycobacterium tuberculosis. Among all the compounds evaluated, fourteen presented MIC values in the range of 0.78 to 6.25 μg/mL against susceptible and resistant
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service