All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5ClN2S
CAS Number:
Molecular Weight:
160.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.6 (lit.)
bp
139-140 °C/36 mmHg (lit.)
mp
−2 °C (lit.)
density
1.381 g/mL at 25 °C (lit.)
functional group
chloro
thioether
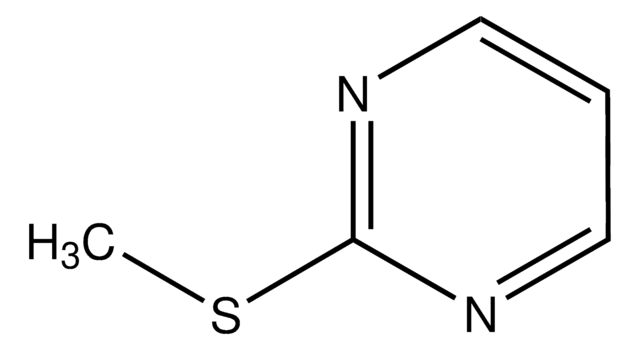
SMILES string
CSc1nccc(Cl)n1
InChI
1S/C5H5ClN2S/c1-9-5-7-3-2-4(6)8-5/h2-3H,1H3
InChI key
DFOHHQRGDOQMKG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Chloro-2-methylthiopyrimidine was used in the total synthesis of the marine alkaloid variolin B1 and 2-hydroxy-4-pyrimidinecarboxylic acid. It was used in the synthesis of 2,4-disubstituted pyrimidines, a novel class of KDR kinase inhibitors. It was used as building block in medicinal chemistry synthesis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pyrimidines. XIII. 2-and 6-substituted 4-pyrimidinecarboxylic acids.
Daves GD, et al.
Journal of Heterocyclic Chemistry, 1(3), 130-133 (1964)
David D Davey et al.
Journal of medicinal chemistry, 50(6), 1146-1157 (2007-02-24)
By the screening of a combinatorial library for inhibitors of nitric oxide (NO) formation by the inducible isoform of nitric oxide synthase (iNOS) using a whole-cell assay, 2-(imidazol-1-yl)pyrimidines were identified. Compounds were found to inhibit the dimerization of iNOS monomers
Peter J Manley et al.
Bioorganic & medicinal chemistry letters, 13(10), 1673-1677 (2003-05-06)
2,4-Disubstituted pyrimidines were synthesized as a novel class of KDR kinase inhibitors. Evaluation of the SAR of the screening lead compound 1 (KDR IC(50)=105 nM, Cell IC(50)=8% inhibition at 500 nM) led to the potent 3,5-dimethylaniline derivative 2d (KDR IC(50)=6
Mark H Norman et al.
Journal of medicinal chemistry, 50(15), 3497-3514 (2007-06-26)
The vanilloid receptor-1 (VR1 or TRPV1) is a member of the transient receptor potential (TRP) family of ion channels and plays a role as an integrator of multiple pain-producing stimuli. From a high-throughput screening assay, measuring calcium uptake in TRPV1-expressing
Regan J Anderson et al.
The Journal of organic chemistry, 70(16), 6204-6212 (2005-07-30)
The total synthesis of the marine alkaloid variolin B has been achieved in 8 steps and 17% overall yield, starting from commercially available 4-chloro-2-methylthiopyrimidine. The key reaction involves the tandem deoxygenation and cyclization of a triarylmethanol using a combination of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)