All Photos(2)
About This Item
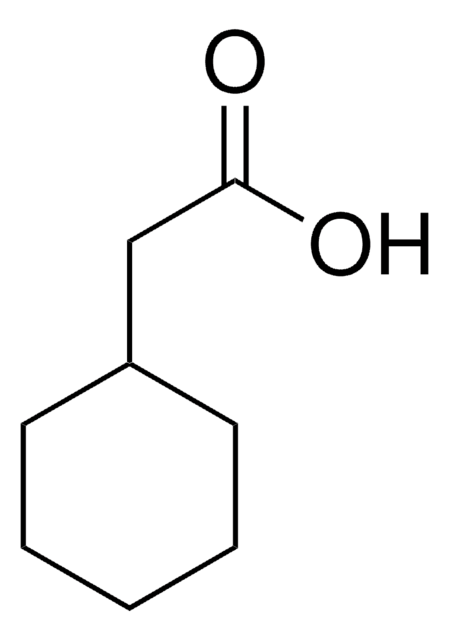
Linear Formula:
CH3C6H10CO2H
CAS Number:
Molecular Weight:
142.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
234 °C (lit.)
mp
36-39 °C (lit.)
functional group
carboxylic acid
SMILES string
CC1(CCCCC1)C(O)=O
InChI
1S/C8H14O2/c1-8(7(9)10)5-3-2-4-6-8/h2-6H2,1H3,(H,9,10)
InChI key
REHQLKUNRPCYEW-UHFFFAOYSA-N
Related Categories
General description
1-Methyl-1-cyclohexanecarboxylic acid is the structural analog of valproic acid and its pharmacokinetic action has been studied in female Sprague-Dawley rats.
Application
1-Methyl-1-cyclohexanecarboxylic acid was used as internal standard during the determination of valproic acid metabolites.
Biochem/physiol Actions
1-Methyl-1-cyclohexanecarboxylic acid is an anticonvulsant drug and causes maturation of murine neuroblastoma cells in vitro.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J C Larcher et al.
Experimental cell research, 203(1), 72-79 (1992-11-01)
Adriamycin, an anticancer agent acting on topoisomerase II, promotes the arrest of cell division and neurite extension in a "neurite-minus" murine neuroblastoma cell line, N1A-103. This morphological differentiation is accompanied by a blockade in the S phase of the cell
J C Larcher et al.
Oncogene, 6(4), 633-638 (1991-04-01)
Using clones N1E-115 and N1A-103 from mouse neuroblastoma C1300, a comparative analysis of c- and N-myc gene expression was undertaken both in proliferating cells and in cultures exposed to conditions which induce differentiation. Under the latter conditions, while N1E-115 cells
A J Sadeque et al.
The Journal of pharmacology and experimental therapeutics, 283(2), 698-703 (1997-11-14)
Cytochrome P450-dependent desaturation of the anticonvulsant drug valproic acid (VPA) results in formation of the hepatotoxin, 4-ene-VPA. Polytherapy with other anticonvulsants which are known P450 inducers increases the flux through this bioactivation pathway. The aim of the present study was
S A Fischkoff et al.
Journal of biological response modifiers, 3(2), 132-137 (1984-01-01)
The anticonvulsant drug 1-methyl-1-cyclohexanecarboxylic acid ( MCCA ) has been shown to cause maturation of murine neuroblastoma cells in vitro at concentrations that are pharmacologically achievable. HL-60 human promyelocytic leukemia cells cultured with this drug underwent a dose-dependent decrease in
J L Vayssiere et al.
Biochemical and biophysical research communications, 140(3), 789-796 (1986-11-14)
CCA, a potent neuroblastoma differentiation inducer, was shown by oxygraphic measurements to reduce significantly the O2 consumption of whole neuroblastoma cells as of mitochondria purified from neuroblastoma or mouse cortex. The effect of CCA on the respiration was compared to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service