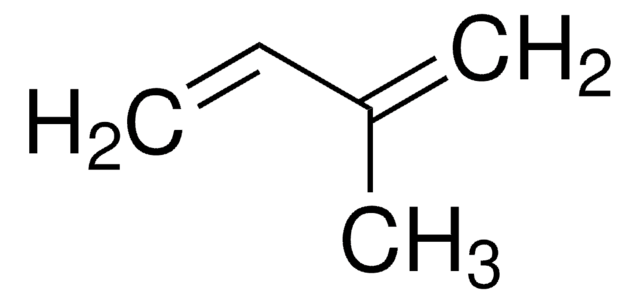
110930
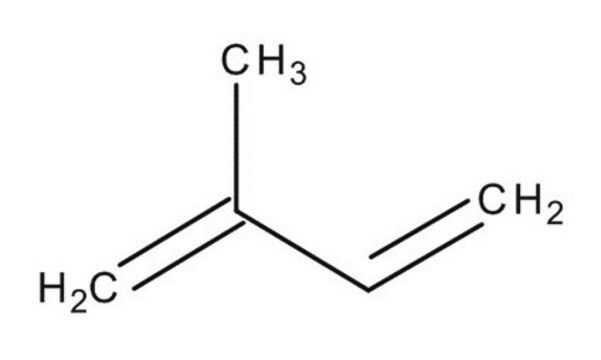
3-Methyl-1,2-butadiene
97%
Synonym(s):
1,1-Dimethylallene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
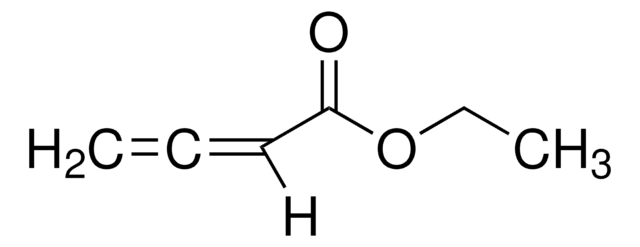
(CH3)2C=C=CH2
CAS Number:
Molecular Weight:
68.12
Beilstein:
1697090
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
6.87 psi ( 20 °C)
Quality Level
Assay
97%
refractive index
n20/D 1.419 (lit.)
bp
40-41 °C (lit.)
mp
−148 °C (lit.)
density
0.694 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)=C=C
InChI
1S/C5H8/c1-4-5(2)3/h1H2,2-3H3
InChI key
PAKGDPSCXSUALC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Methyl-1,2-butadiene (1,1-dimethylallene) has been used to study the photo-induced reaction of 1,1-dimethylallene with the cyanoarenes 1,2,4,5-tetracyanobenzene, 1,4-dicyanobenzene and 1,4-dicyanonaphthalene in the presence of methanol as nucleophile.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
10.4 °F - closed cup
Flash Point(C)
-12 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The electron transfer photochemistry of allenes with cyanoarenes. Photochemical nucleophile?olefin combination, aromatic substitution (photo-NOCAS) and related reactions.
Mangion D, et al.
J. Chem. Soc. Perkin Trans. II, 1, 48-60 (2001)
Shaochen Zhang et al.
Science (New York, N.Y.), 364(6435), 45-51 (2019-04-06)
Accessing enantiomerically enriched amines often demands oxidation-state adjustments, protection and deprotection processes, and purification procedures that increase cost and waste, limiting applicability. When diastereomers can be formed, one isomer is attainable. Here, we show that nitriles, largely viewed as insufficiently
Global Trade Item Number
| SKU | GTIN |
|---|---|
| M48805-100G | |
| M48805-100ML | 4061834059271 |
| M48805-500ML | |
| M48805-5G | |
| W270709-1KG | |
| W270709-20KG | |
| W270709-800G | |
| W270709-8KG-K | 4061837516160 |
| W270709-SAMPLE-K | 4061838180889 |
| W270733-SAMPLE | |
| M48805-5ML | 4061834059288 |
| 67397-1ML | 4061832735559 |
| M48805-500G | |
| W270709-1KG-K | 4061838249067 |
| W270709-20KG-K | 4061838180865 |
| W270709-800G-K | |
| W270709-8KG | |
| W270709-SAMPLE | |
| W270733-100G | |
| W270733-100G-K | 4061838180896 |
| W270733-1KG | |
| W270733-1KG-K | 4061838180902 |
| W270733-SAMPLE-K | 4061838180926 |
| W270733-25G | |
| W270733-25G-K | 4061838180919 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)