L7269
α-Lactalbumin from human milk
≥95% (SDS-PAGE), lyophilized powder
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
human milk
Quality Level
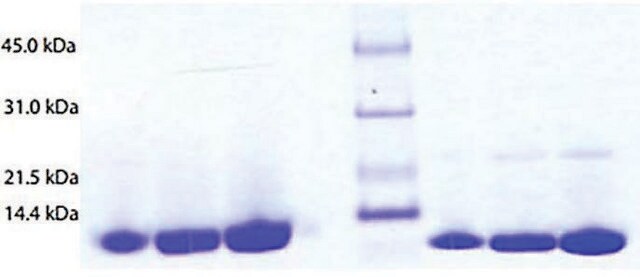
Assay
≥95% (SDS-PAGE)
form
lyophilized powder
mol wt
14,070 Da by calculation
solubility
H2O: soluble 10 mg/mL(lit.)
UniProt accession no.
storage temp.
−20°C
Gene Information
human ... LALBA(3906)
Looking for similar products? Visit Product Comparison Guide
General description
α-Lactalbumin (α-LA) is a small, acidic, whey protein that constitutes about 22% of the total proteins in human milk. It is produced by the epithelial cells of the mammary gland. α-LA is made up of two domains, a large α-helical domain, and a small β-sheet domain.
Application
α-Lactalbumin (α-LA) has been used as a standard
- to study the partitioning behavior of different monomeric proteins with exposure to amino acids on the protein surface
- to study the interaction between α-LA and cathepsin D
- to study the ability of breast milk fractions to enhance the transepithelial flux of extrinsic iron in colon carcinoma cell line
Biochem/physiol Actions
α-Lactalbumin (α-LA) forms a complex with lactose synthase within the mammary gland and plays a role in milk production and regulates milk volume. It acts as an essential source for bioactive peptides and essential amino acids such as lysine, tryptophan, branched-chain amino acids, and sulfur-containing amino acids that play a role in an infant′s nutrition. In addition, α-LA has a wide range of applications including a supplement to foster gastrointestinal health and modulate sleep and depression. α-LA also shows therapeutic effects against sarcopenia, seizures, mood disorders, and cancer. It has a Ca2+ binding site that binds with Na+, K+, Mg2+, and Mn2+ and many Zn2+ binding sites.
Alters the substrate specificity of galactosyltransferase to increase the rate of lactose formation; the complex of galactosyltransferase and α-lactalbumin is called lactose synthase. Complexes of α-lactalbumin with oleic acid show drastically different activities than α-lactalbumin alone, being strongly cytotoxic to tumor cells. The complex is referred to as HAMLET (human alpha-lactalbumin made lethal to tumor cells).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Breast Milk Fractions Solubilize Fe(III) and Enhance Iron Flux across Caco-2 Cells
Robert E. S.
The Journal of Nutrition, 449?455-449?455 (2003)
Junai Gan et al.
Molecular nutrition & food research, 63(18), e1900259-e1900259 (2019-07-05)
The use of human milk products is increasing for high-risk infants. Human milk contains endogenous enzymes that comprise a dynamic proteolytic system, yet biological properties of these enzymes and their activities in response to variations including pH within infants are
Antonio Carroccio et al.
Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 8(3), 254-260 (2009-11-26)
A percentage of patients with symptoms of irritable bowel syndrome (IBS) suffer from food hypersensitivity (FH) and improve on a food-elimination diet. No assays have satisfactory levels of sensitivity for identifying patients with FH. We evaluated the efficacy of an
Comparison of the amino acid sequence of bovine alpha-lactalbumin and hens egg white lysozyme.
K Brew et al.
The Journal of biological chemistry, 242(16), 3747-3749 (1967-08-25)
E A Permyakov et al.
FEBS letters, 473(3), 269-274 (2000-05-20)
Small milk protein alpha-lactalbumin (alpha-LA), a component of lactose synthase, is a simple model Ca(2+) binding protein, which does not belong to the EF-hand proteins, and a classical example of molten globule state. It has a strong Ca(2+) binding site
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service