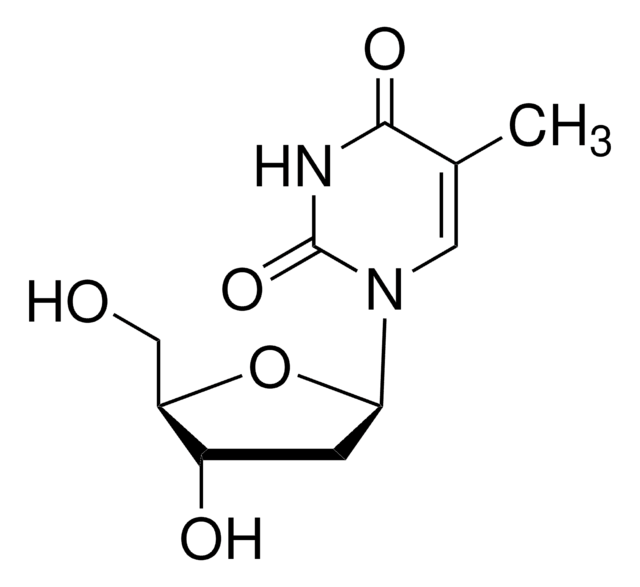
E9386
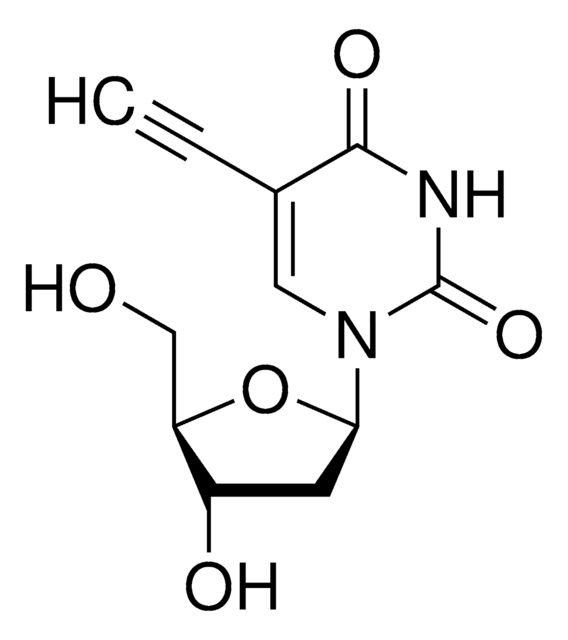
5-Ethyl-2′-deoxyuridine
Synonym(s):
EUdR, Edoxudine
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥95% (HPLC)
form
powder
solubility
water: 50 mg/mL, clear, colorless to yellow
storage temp.
−20°C
SMILES string
CCC1=CN(C2CC(O)C(CO)O2)C(=O)NC1=O
InChI
1S/C11H16N2O5/c1-2-6-4-13(11(17)12-10(6)16)9-3-7(15)8(5-14)18-9/h4,7-9,14-15H,2-3,5H2,1H3,(H,12,16,17)
InChI key
XACKNLSZYYIACO-UHFFFAOYSA-N
Biochem/physiol Actions
Linkage
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service