D4681
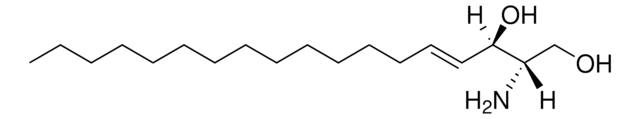
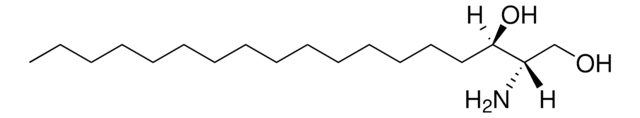
L-threo-Dihydrosphingosine
≥95% (TLC)
Synonym(s):
Safingol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H39NO2
CAS Number:
Molecular Weight:
301.51
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (TLC)
form
powder
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCCCC[C@H](O)[C@@H](N)CO
InChI
1S/C18H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h17-18,20-21H,2-16,19H2,1H3/t17-,18-/m0/s1
InChI key
OTKJDMGTUTTYMP-ROUUACIJSA-N
Biochem/physiol Actions
Sphingosine kinase inhibitor; protein kinase C alpha (PKCα) -specific inhibitor; Sphingosine analog; potentiates the effect of doxorubicin (DOX) in tumor-bearing animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G K Schwartz et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 3(4), 537-543 (1997-04-01)
We performed a pilot clinical trial with safingol (L-threo-dihydrosphingosine), a protein kinase C-specific inhibitor that potentiates the effect of doxorubicin (DOX) in tumor-bearing animals. Safingol was initially administered as a 1-h infusion at escalating doses. Fourteen days later, patients received
Yi-Hsin Hsu et al.
Cancer research, 74(17), 4822-4835 (2014-06-28)
Triple-negative breast cancer (TNBC) is a highly heterogeneous and recurrent subtype of breast cancer that lacks an effective targeted therapy. To identify candidate therapeutic targets, we profiled global gene expression in TNBC and breast tumor-initiating cells with a patient survival
J W Darges et al.
Advances in experimental medicine and biology, 400A, 387-392 (1997-01-01)
The sphingosine analog L-threo-dihydrosphingosine has been shown to inhibit protein kinase C (PKC) isoenzymes in mixed micelle and vesicle assays. This compound also inhibited the reactive oxygen intermediates (ROI) released from isolated neutrophils (IC50 approximately 2 microM) and phorbol ester-induced
Emma M Dangerfield et al.
Chembiochem : a European journal of chemical biology, 13(9), 1349-1356 (2012-05-29)
The immunomodulatory glycolipid α-galactosylceramide (α-GalCer) binds to CD1d and exhibits potent activity as a ligand for invariant CD1d-restricted natural killer-like T cells (iNKT cells). Structural analogues of α-GalCer have been synthesised to determine which components are required for CD1d presentation
Yidi Sun et al.
Molecular biology of the cell, 23(12), 2388-2398 (2012-04-27)
Sphingoid intermediates accumulate in response to a variety of stresses, including heat, and trigger cellular responses. However, the mechanism by which stress affects sphingolipid biosynthesis has yet to be identified. Recent studies in yeast suggest that sphingolipid biosynthesis is regulated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service