C9268
Carboxypeptidase A from bovine pancreas
(Type II-PMSF treated), ≥50 units/mg protein, ready-to-use solution
Synonym(s):
Carboxypolypeptidase, Peptidyl-L-amino-acid hydrolase
About This Item
Recommended Products
grade
Proteomics Grade
Quality Level
form
ready-to-use solution
quality
(Type II-PMSF treated)
specific activity
≥50 units/mg protein
mol wt
~35 kDa
purified by
2× crystallization
impurities
≤0.05 BTEE units/mg protein chymotrypsin
≤10 BAEE units/mg protein trypsin
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
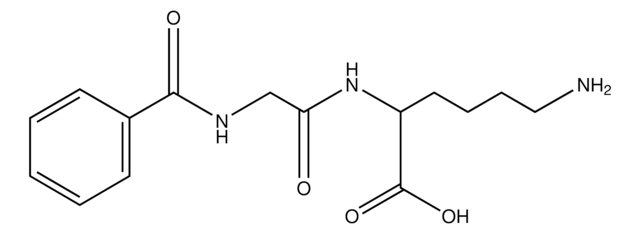
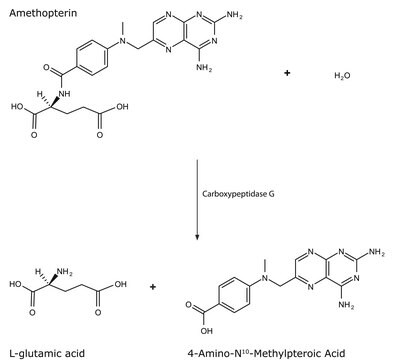
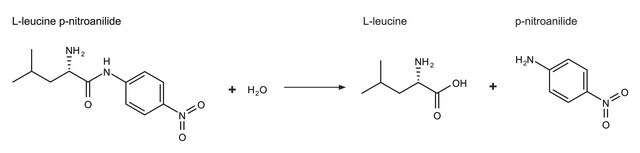
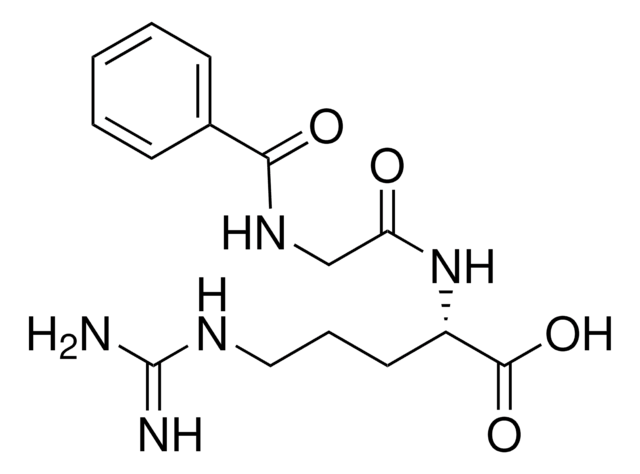
Unit Definition
Preparation Note
Analysis Note
inhibitor
substrate
Signal Word
Danger
Hazard Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Objective: To standardize a procedure for the assay of Carboxypeptidase A
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service