A6888
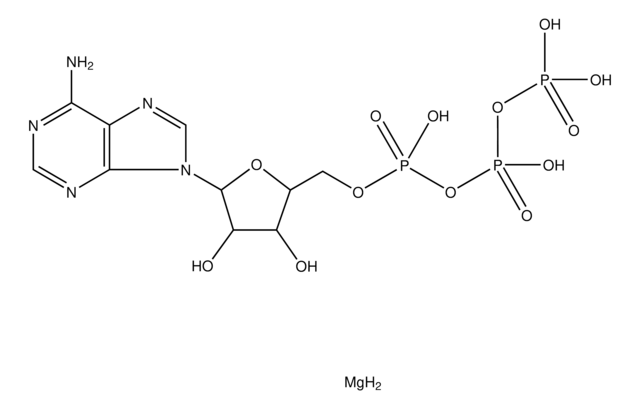
Adenosine 5′-triphosphate–Agarose
aqueous glycerol suspension
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
aqueous glycerol suspension
extent of labeling
≥1 μmol per mL
matrix
cross-linked 4% beaded agarose
matrix activation
cyanogen bromide
matrix attachment
ribose hydroxyls
matrix spacer
11 atoms (adipic acid dihydrazide)
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Adenosine 5′-triphosphate Agarose (5′-ATP agarose) has been used in affinity chromatography to purify uridine kinase from Ehrlich ascites tumor cells.
Physical form
Suspension in 50% glycerol containing 0.25 M NaCl
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B L Stitt
The Journal of biological chemistry, 263(23), 11130-11137 (1988-08-15)
We have determined that 3 mol of ATP or other adenine nucleotide can bind to Escherichia coli transcription termination protein rho, in the presence or absence of the RNA cofactor that is required for activation of rho's ATPase activity. Isotope
S Ogg et al.
The Journal of biological chemistry, 269(48), 30461-30469 (1994-12-02)
Human Cdc25C is a protein phosphatase that dephosphorylates and activates Cdc2-cyclin B to trigger entry into mitosis. Cdc25C is itself regulated by phosphorylation. In asynchronously growing HeLa cells, we have determined that serine 216 is the major site of Cdc25C
V Nagaraja et al.
Journal of molecular biology, 182(4), 579-587 (1985-04-20)
We have purified the type I restriction enzymes SB and SP from Salmonella typhimurium and S. potsdam, respectively, and determined the DNA sequences that they recognize. These sequences resemble those previously determined for the type I enzymes, EcoB, EcoK and
H D Kim et al.
Biochemistry, 38(44), 14697-14710 (1999-11-05)
Two polynucleotide-dependent ATPases, 95 and 181 kDa in size, have been purified to near homogeneity from cell-free extracts of Schizosaccharomyces pombe. Despite their size differences, their biochemical properties were strikingly similar. Both enzymes were capable of unwinding RNA and DNA
B Suri et al.
The EMBO journal, 3(3), 575-579 (1984-03-01)
The EcoA restriction enzyme from Escherichia coli 15T- has been isolated. It proves to be an unusual enzyme, clearly related functionally to the classical type I restriction enzymes. The basic enzyme is a two subunit modification methylase. Another protein species
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service