A1509
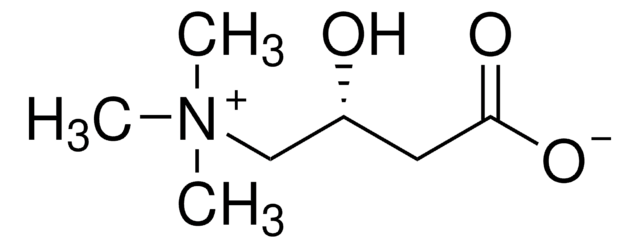
Acetyl-DL-carnitine hydrochloride
>98% (TLC)
Synonym(s):
(±)-3-Acetoxy-4-(trimethylammonio)butyrate hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H17NO4 · HCl
CAS Number:
Molecular Weight:
239.70
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Acetyl-DL-carnitine hydrochloride,
Assay
>98% (TLC)
form
powder
color
white
storage temp.
−20°C
SMILES string
Cl.CC(=O)OC(CC(O)=O)C[N](C)(C)C
InChI
1S/C9H18NO4.ClH/c1-7(11)14-8(5-9(12)13)6-10(2,3)4;/h8H,5-6H2,1-4H3,(H,12,13);1H
InChI key
KEOLEGXENCLAMX-UHFFFAOYSA-N
Biochem/physiol Actions
Weak cholinergic receptor agonist; intermediate in lipid metabolism.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P D Prasad et al.
Biochimica et biophysica acta, 1284(1), 109-117 (1996-10-02)
The JAR human placental choriocarcinoma cells were found to transport carnitine into the intracellular space by a Na(+)-dependent process. The transport showed no requirement for anions. The Na+-dependent process was saturable and the apparent Michaelis-Menten constant for carnitine was 12.3
J M Mauro et al.
The Biochemical journal, 237(2), 533-540 (1986-07-15)
A photolabile reagent, p-azidophenacyl-DL-thiocarnitine, was synthesized and tested as a photoaffinity label for carnitine acetyltransferase (EC 2.3.1.7) from pigeon breast. p-Azidophenacyl-DL-thiocarnitine is an active-site-directed reagent for this acetyltransferase, since it is a competitive inhibitor (Ki 10 microM) versus carnitine. U.v.
A S Alhomida et al.
Biochimie, 78(3), 204-208 (1996-01-01)
The kinetic properties of carnitine acetyltransferase from the skeletal muscle of the Arabian camel (Camelus dromedarius) were studied. The enzyme showed an optimum pH between 7.2 and 8.2. Reciprocal plots of data obtained by varying one substrate concentration while keeping
Anna Bogusiewicz et al.
The Journal of nutrition, 145(1), 32-40 (2014-12-21)
A large number of birth defects are related to nutrient deficiencies; concern that biotin deficiency is teratogenic in humans is reasonable. Surprisingly, studies indicate that increased urinary 3-hydroxyisovalerylcarnitine (3HIAc), a previously validated marker of biotin deficiency, is not a valid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service