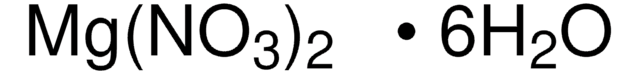63084
Magnesium nitrate hexahydrate
BioUltra, ≥99.0% (KT)
Synonym(s):
Magnesium dinitrate hexahydrate, Magnesium nitrate hydrate, Mg(NO3)2.6H2O
About This Item
Recommended Products
vapor density
6 (vs air)
Quality Level
product line
BioUltra
Assay
≥99.0% (KT)
form
solid
impurities
insoluble matter, passes filter test
pH
5.0-8.2 (25 °C, 1 M in H2O)
mp
89 °C (dec.) (lit.)
solubility
H2O: 1 M at 20 °C, clear, colorless
anion traces
chloride (Cl-): ≤50 mg/kg
sulfate (SO42-): ≤50 mg/kg
cation traces
Al: ≤5 mg/kg
As: ≤0.1 mg/kg
Ba: ≤10 mg/kg
Bi: ≤10 mg/kg
Ca: ≤5000 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤100 mg/kg
Li: ≤5 mg/kg
Mn: ≤5 mg/kg
Mo: ≤5 mg/kg
Na: ≤100 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Sr: ≤10 mg/kg
Zn: ≤5 mg/kg
absorption
cut-off at 333 nm in H2O at 1 M
SMILES string
O.O.O.O.O.O.[Mg++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Mg.2NO3.6H2O/c;2*2-1(3)4;;;;;;/h;;;6*1H2/q+2;2*-1;;;;;;
InChI key
MFUVDXOKPBAHMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Thermal energy storage: Research highlights the use of magnesium nitrate hexahydrate as a salt hydrate phase change material for thermal energy storage in printing composites, indicating its potential in enhancing the efficiency of energy storage systems (Lak et al., 2023).
- Encapsulation in non-aqueous emulsions: A study on the encapsulation of hygroscopic liquids mentions the utilization of magnesium nitrate hexahydrate, focusing on its stability and performance in controlled release applications, which are critical in various industrial and pharmaceutical processes (Lak et al., 2022).
- Supramolecular metallogel for separation processes: Utilization of magnesium nitrate hexahydrate in the formation of a thixotropic supramolecular metallogel is reported, showcasing its application in iodine sequestration and column-based dye separation, highlighting its versatility in purification and chemical separation technologies (Alam and Sarma, 2020).
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service