44920
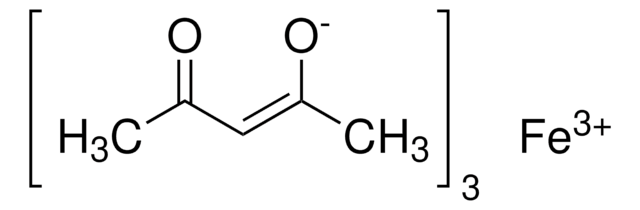
Iron(III) acetylacetonate
purum, ≥97.0% (RT)
Synonym(s):
2,4-Pentanedione iron(III) derivative, Fe(acac)3, Ferric acetylacetonate, Iron(III) 2,4-pentanedionate
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥97.0% (RT)
form
powder
reaction suitability
core: iron
reagent type: catalyst
mp
180-182 °C (dec.) (lit.)
180-190 °C (dec.)
density
5.24 g/mL at 25 °C (lit.)
SMILES string
CC(=O)\C=C(\C)O[Fe](O\C(C)=C/C(C)=O)O\C(C)=C/C(C)=O
InChI
1S/3C5H8O2.Fe/c3*1-4(6)3-5(2)7;/h3*3,6H,1-2H3;/q;;;+3/p-3/b3*4-3-;
InChI key
AQBLLJNPHDIAPN-LNTINUHCSA-K
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A precursor in the synthesis of Fe3O4/carbon composite fibers via forcespinning. This fiber material is suitable for use as anode materials in lithium-ion batteries.
- A precursor in the synthesis of iron-based metal-organic frameworks (MOFs) for the applications in rechargeable alkali-ion batteries.
- A catalyst in the controlled living radical polymerization of vinyl acetate to synthesis of polymers with controlled molecular weights.
- A precursor to synthesize iron-doped titania (Fe-TiO2) photocatalysts for the potential applications in photocatalytic reactions.
- A MOCVD precursor for highly crystalline (Zn,Fe)Fe2O4 films and magnetic property measurements of these films.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service