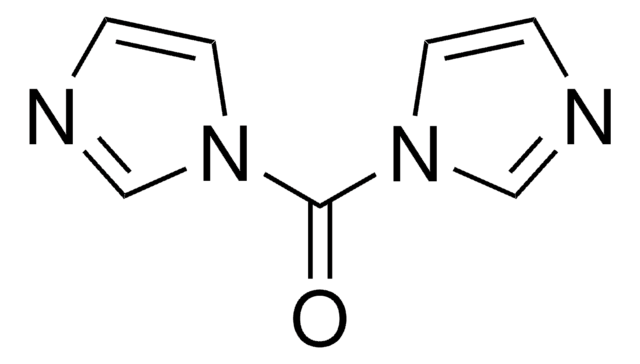
43720
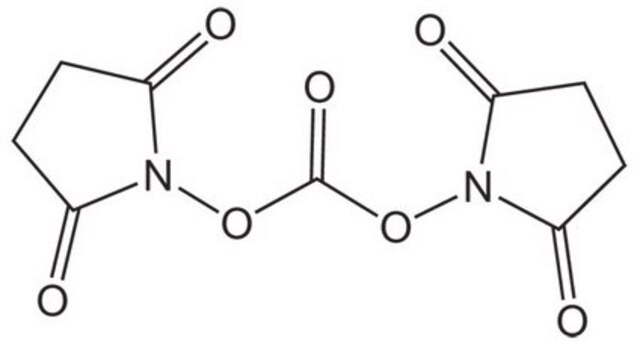
N,N′-Disuccinimidyl carbonate
≥95.0% (NMR), for peptide synthesis
Synonym(s):
N-Succinimidyl carbonate, DSC, Di(N-succinimidyl) carbonate
About This Item
Recommended Products
Product Name
N,N′-Disuccinimidyl carbonate, purum, ≥95.0% (NMR)
grade
purum
Quality Level
Assay
≥95.0% (NMR)
form
powder
reaction suitability
reaction type: Carbonylations
impurities
~3% N-hydroxysuccinimide (NMR)
mp
190 °C (dec.) (lit.)
application(s)
peptide synthesis
functional group
imide
storage temp.
−20°C
SMILES string
O=C1CCC(=O)N1OC(=O)ON2C(=O)CCC2=O
InChI
1S/C9H8N2O7/c12-5-1-2-6(13)10(5)17-9(16)18-11-7(14)3-4-8(11)15/h1-4H2
InChI key
PFYXSUNOLOJMDX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Various carbamate derivatives from primary and sterically hindered secondary alcohols by alkoxycarbonylation.
- Active carbonate resins from 4-hydroxymethylpolystyrene and 4-hydroxymethyl-3-nitrobenzamido resins via hydroxy functional groups.
- Aza-glycinyl dipeptides, important intermediates for the preparation of various azapeptides.
It may be also used:
- In the two-step preparation of 5-(6-(azidomethyl)nicotinamido)pentanoic acid, a copper-chelating picolyl azide derivative.
- To activate the hydroxyl group of the hapten, γ-hydroxyphenylbutazone (HPBZ) so that HPBZ can effectively bind with human serum albumin(HSA)-immunogen to form a hapten-protein conjugate.
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service