42579
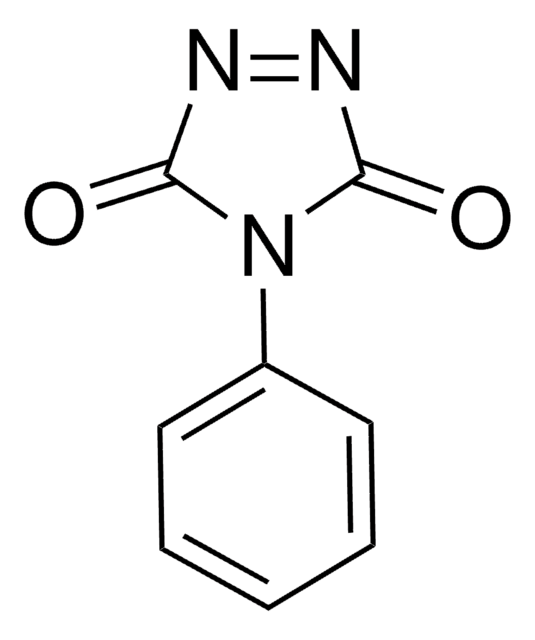
4-Phenyl-1,2,4-triazoline-3,5-dione
for HPLC derivatization, LiChropur™, ≥98.0% (CHN)
Synonym(s):
Cookson reagent, 4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione, PTAD
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H5N3O2
CAS Number:
Molecular Weight:
175.14
Beilstein:
141548
EC Number:
MDL number:
UNSPSC Code:
41116105
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
for HPLC derivatization
Quality Level
Assay
≥98.0% (CHN)
quality
LiChropur™
technique(s)
HPLC: suitable
mp
165-170 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
O=C1N=NC(=O)N1c2ccccc2
InChI
1S/C8H5N3O2/c12-7-9-10-8(13)11(7)6-4-2-1-3-5-6/h1-5H
InChI key
ISULLEUFOQSBGY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
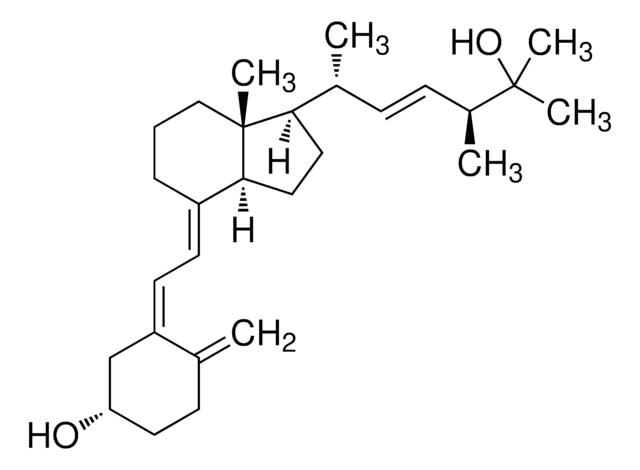
4-Phenyl-1,2,4-triazoline-3,5-dione, also known as Cookson reagent, is a strong dienophile, which gives a stable Diels-Alder adduct quantitatively within a short time and under mild conditions. It is commonly used as a protecting group of the diene moiety for the synthesis of vitamin D3 (VD3)-related compounds.
Application
4-Phenyl-1,2,4-triazoline-3,5-dione may be used as a derivatizing reagent for the determination of trace levels of 25-hydroxyvitamin D and its C-3 epimer in biological samples and cholecalciferol (vitamin D3) in fortified infant formula, milk and milk powder using liquid chromatography–tandem mass spectrometry (LC–MS/MS)technique.
Legal Information
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatographic separation of PTAD-derivatized 25-hydroxyvitamin D3 and its C-3 epimer from human serum and murine skin
Teegarden DM, et al.
Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 991(1), 118-121 (2015)
Grant A Abernethy
Analytical and bioanalytical chemistry, 403(5), 1433-1440 (2012-03-24)
A method for analysing vitamin D(3) (VD3, cholecalciferol) has been established and validated. This method is rapid and cost effective and is intended for use in quality control in the manufacture of fortified infant formulae and milk powders. Milk or
A S Weiskopf et al.
Journal of mass spectrometry : JMS, 36(1), 71-78 (2001-02-17)
The structural specificity of vitamin D derivatization by PTAD (4-phenyl-1,2,4-triazoline-3,5-dione) was probed using synthetic analogues and ion trap mass spectrometry. EB 1089, a vitamin D(3) analogue which contains a second site for Diels--Alder cycloaddition on its side-chain, allowed the examination
Jiri Adamec et al.
Journal of separation science, 34(1), 11-20 (2010-12-21)
Simultaneous and accurate measurement of vitamin D and 25-hydroxyvitamin D in biological samples is a barrier limiting our ability to define "optimal" vitamin D status. Thus, our goal was to optimize conditions and evaluate an LC-MS method for simultaneous detection
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service