860526P
Avanti
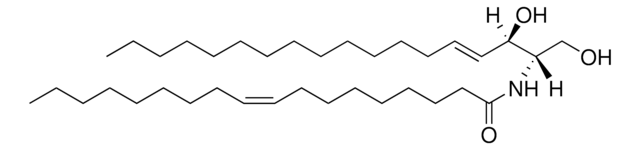
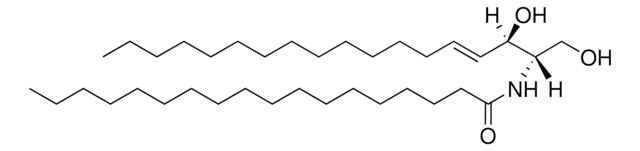
1-O-Acyl-Ceramide
Avanti Polar Lipids 860526P, powder
Synonym(s):
1-oleoyl-N-heptadecanoyl-D-erythro-sphingosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C53H101NO4
CAS Number:
Molecular Weight:
816.37
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 5 mg (860526P-5mg)
manufacturer/tradename
Avanti Polar Lipids 860526P
lipid type
sphingolipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
[H][C@](/C=C/CCCCCCCCCCCCC)(O)[C@@]([H])(NC(CCCCCCCCCCCCCCCC)=O)COC(CCCCCCC/C=C\CCCCCCCC)=O
General description
1-O-Acyl-Ceramide is an epidermal ceramide present in mouse and human epidermis. It comprises long chain fatty acids in 1-O-position and is synthesized in the endoplasmic reticulum-related sites.
Application
1-O-Acyl-Ceramide is suitable for use as a lipid standard in
- high performance thin layer chromatography (HPTLC)
- in mass and nuclear magnetic resonance spectroscopy for the quantification of lipids isolated from vernix caseosa skin cream
- in liquid chromatography-tandem mass spectrometry for the quantification of 1-O-Acyl ceramides from liver samples
Biochem/physiol Actions
1-O-Acyl-Ceramide functions in maintaining water barrier homeostasis. Deficiency of the glucosylceramide synthase enzyme leads to the accumulation of O-acylceramides. Treatment of human monoclonal antibody, REMD 2.59 leads to the accumulation of 1-O-acyl-ceramides in soleus muscle and depletion in liver.
Packaging
5 mL Amber Glass Screw Cap Vial (860526P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Glucagon receptor antagonism improves glucose metabolism and cardiac function by promoting AMP-mediated protein kinase in diabetic mice
Sharma AX, et al.
Testing, 22(7), 1760-1773 (2018)
Nonhydroxylated 1-O-acylceramides in vernix caseosa
Harazim E, et al.
Journal of Lipid Research, 59(11), 2164-2173 (2018)
1-O-acylceramides are natural components of human and mouse epidermis
Rabionet M, et al.
Journal of Lipid Research, 54(12), 3312-3321 (2013)
Tamoxifen inhibits the biosynthesis of inositolphosphorylceramide in Leishmania
Trinconi CT, et al.
International Journal for Parasitology, Drugs and Drug Resistance, 8(3), 475-487 (2018)
Akira Abe et al.
Journal of lipid research, 48(10), 2255-2263 (2007-07-14)
A novel lysosomal phospholipase A(2) (LPLA2) with specificity toward phosphatidylethanolamine and phosphatidylcholine was previously purified and cloned. LPLA2 transfers sn-1 or sn-2 acyl groups of phospholipids to the C1 hydroxyl of the short-chain ceramide N-acetylsphingosine (NAS) under acidic conditions. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service