P23954
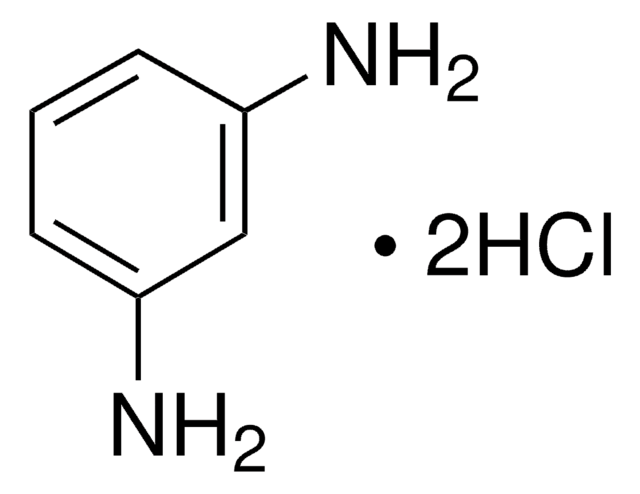
m-Phenylenediamine
flakes, 99%
Synonym(s):
1,3-Benzenediamine, 1,3-Diaminobenzene, 1,3-Phenylenediamine, MPDA
About This Item
Recommended Products
vapor density
3.7 (vs air)
Quality Level
vapor pressure
0.62 mmHg ( 100 °C)
Assay
99%
form
flakes
autoignition temp.
1040 °F
bp
282-284 °C
mp
64-66 °C
SMILES string
Nc1cccc(N)c1
InChI
1S/C6H8N2/c7-5-2-1-3-6(8)4-5/h1-4H,7-8H2
InChI key
WZCQRUWWHSTZEM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- intrinsically electrically semiconducting microparticles of semiladder poly(m-phenylenediamine-co-2-hydroxy-5-sulfonic aniline) structures
- extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) Fe3O4 nanocomposite, used as sorbent for the magnetic solid-phase extraction (MSPE) of polychlorinated biphenyls
- series of terpolymers, via chemical oxidative polymerization
- thin film composite (TFC) membranes based polyamide
- TFC reverse osmosis (RO) membranes
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Muta. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| P23954-500G | 4061834363026 |
| P23954-25G | 4061832768939 |
| P23954-100G | 4061834363019 |
| P23954-15KG | 4061832768922 |
| P23954-1KG | 4061826220320 |
| P23954-5G | 4061834363033 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service