900692
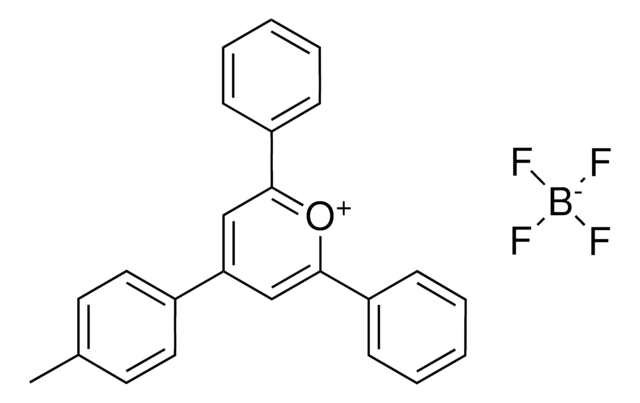
2,4,6-Tris(4-methoxyphenyl)pyrylium tetrafluoroborate
Synonym(s):
2,4,6-Tri-p-anisylpyrylium (TAP) fluoroborate
About This Item
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: catalyst
reaction type: Photocatalysis
mp
346-351 °C
SMILES string
COC(C=C1)=CC=C1C2=[O+]C(C3=CC=C(OC)C=C3)=CC(C4=CC=C(OC)C=C4)=C2.FB(F)F.[F-]
Related Categories
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Cyclization–endoperoxidation cascade reactions of dienes mediated by a pyrylium photoredox catalyst
Metal-Free Ring-Opening Metathesis Polymerization
Cationic Polymerization of Vinyl Ethers Controlled by Visible Light
Electron-Transfer-Induced Diels ± Alder Reactions of Indole and Exocyclic Dienes: Synthesis and Quantum-Chemical Studies
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
The Nicewicz lab is focused on the discovery of new and powerful reaction methodologies that proceed via the intermediacy of highly reactive cation radical species. Included in these transformations are anti-Markovnikov selective additions of amines, alcohols, carboxylic acids, amides and mineral acids to alkenes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service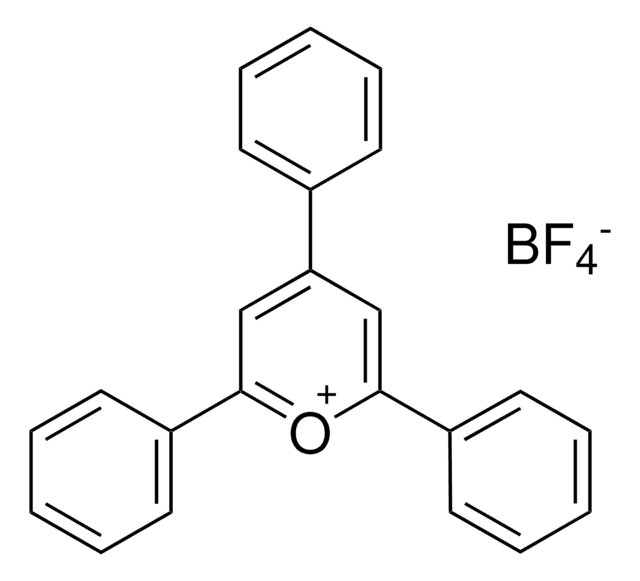
![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)