All Photos(1)
About This Item
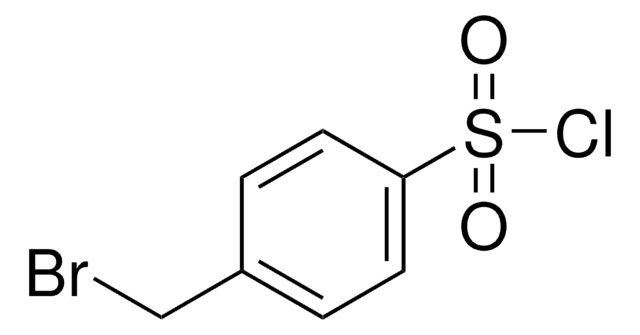
Linear Formula:
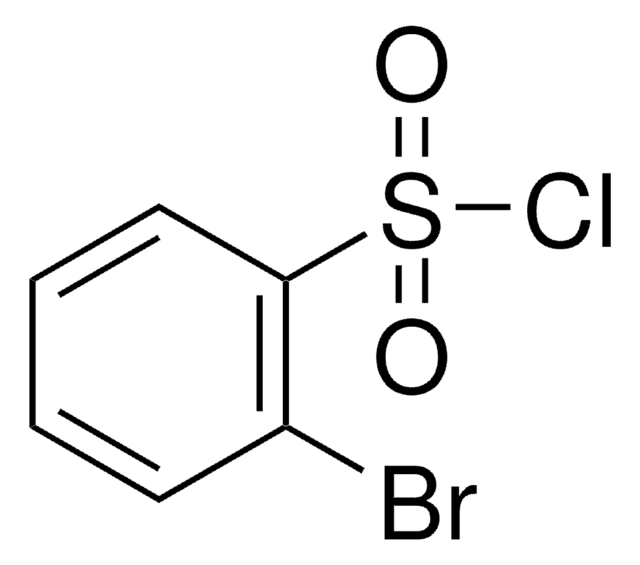
BrC6H4SO2Cl
CAS Number:
Molecular Weight:
255.52
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.593 (lit.)
bp
90-91 °C/0.5 mmHg (lit.)
mp
30-33 °C
density
1.773 g/mL at 25 °C (lit.)
functional group
bromo
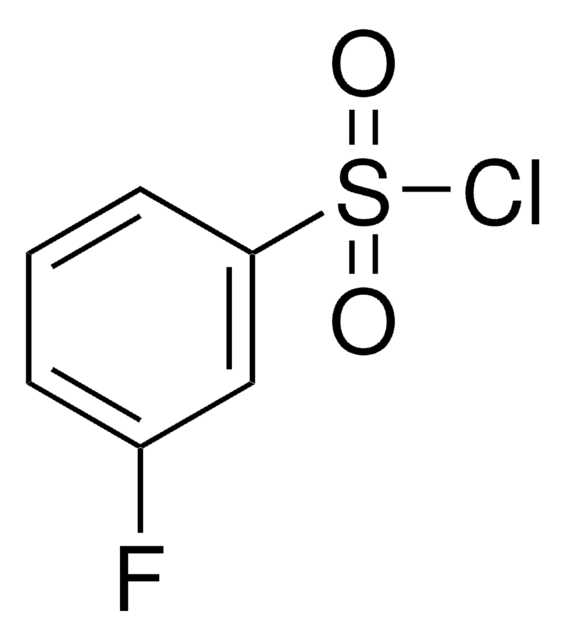
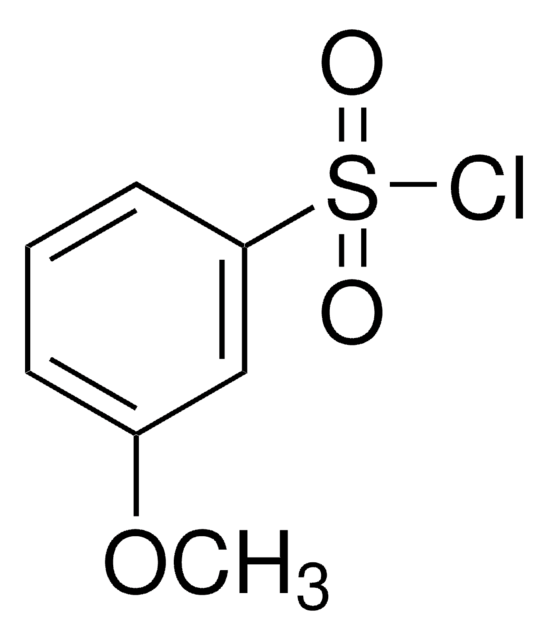
SMILES string
ClS(=O)(=O)c1cccc(Br)c1
InChI
1S/C6H4BrClO2S/c7-5-2-1-3-6(4-5)11(8,9)10/h1-4H
InChI key
PJGOLCXVWIYXRQ-UHFFFAOYSA-N
General description
3-Bromobenzenesulfonyl chloride is an aryl sulfonyl chloride derivative. It participates in the synthesis of N-sulfonylanthranilic acid derivatives and potent P1′ benzenesulfonyl azacyclic urea human immunodeficiency virus (HIV) protease inhibitors.
Application
3-Bromobenzenesulfonyl chloride may be used in the preparation of:
- 2-(3-bromophenyl)-5-n-butylfuran
- 2-(3-bromophenyl)-3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran
- 3-bromo-4-(3-bromophenyl)thiophene
- 2,5-bis(3-bromophenyl)-1-methylpyrrole
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pd?Catalysed Direct Arylation of Heteroaromatics Using (Poly) halobenzenesulfonyl Chlorides as Coupling Partners: One Step Access to (Poly) halo?Substituted Bi (hetero) aryls.
Skhiri A, et al.
European Journal of Organic Chemistry, 2015(20), 4428-4436 (2015)
Peggy P Huang et al.
Bioorganic & medicinal chemistry letters, 14(15), 4075-4078 (2004-07-01)
A series of novel azacyclic urea HIV protease inhibitors bearing a benzenesulfonamide group at P1' were synthesized utilizing a parallel synthesis method. Structural studies of early analogs bound in the enzyme active site were used to design more potent inhibitors.
Zheng Yin et al.
Journal of medicinal chemistry, 52(24), 7934-7937 (2009-12-18)
A novel class of compounds containing N-sulfonylanthranilic acid was found to specifically inhibit dengue viral polymerase. The structural requirements for inhibition and a preliminary structure-activity relationship are described. A UV cross-linking experiment was used to map the allosteric binding site
Articles
Aryl sulfonyl chloride derivatives are frequently used in parallel synthesis to synthesize sulfonamides and sulfonate linkages.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service