47587
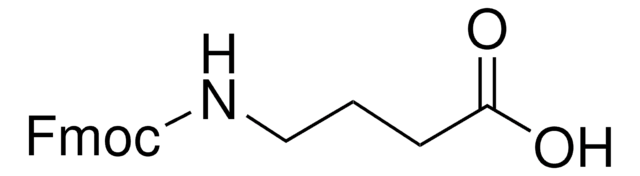
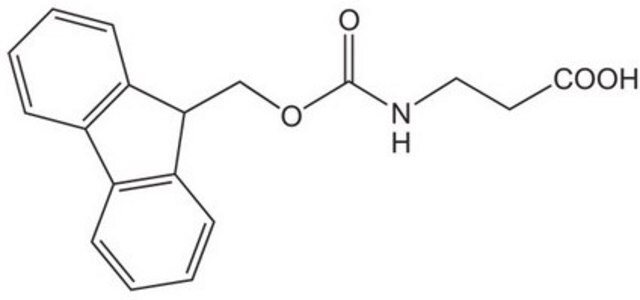
Fmoc-β-Ala-OH
≥99.0% (HPLC)
Synonym(s):
Fmoc-β-alanine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H17NO4
CAS Number:
Molecular Weight:
311.33
Beilstein:
2302327
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
Assay
≥99.0% (HPLC)
form
powder or crystals
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
mp
142-147 °C
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
SMILES string
OC(=O)CCNC(=O)OCC1c2ccccc2-c3ccccc13
InChI
1S/C18H17NO4/c20-17(21)9-10-19-18(22)23-11-16-14-7-3-1-5-12(14)13-6-2-4-8-15(13)16/h1-8,16H,9-11H2,(H,19,22)(H,20,21)
InChI key
LINBWYYLPWJQHE-UHFFFAOYSA-N
General description
Fmoc-β-Ala-OH also known as Fmoc-β-alanine, is a versatile reagent for solid phase peptide synthesis.
Application
Fmoc-β-Ala-OH is used as a linker to synthesize peptides on a monolithic GMA-EDMA (glycidyl methacrylate-co-ethylene dimethacrylate) matrix via Fmoc solid phase peptide synthesis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sofie Trier et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 329-337 (2015-09-09)
Acylation of peptide drugs with fatty acid chains has proven beneficial for prolonging systemic circulation, as well as increasing enzymatic stability and interactions with lipid cell membranes. Thus, acylation offers several potential benefits for oral delivery of therapeutic peptides, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service