418293
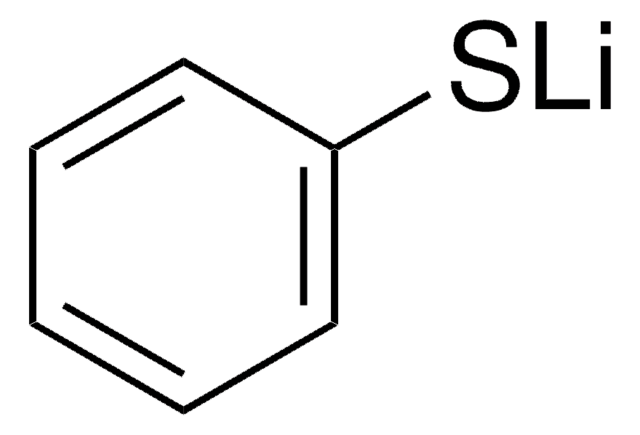
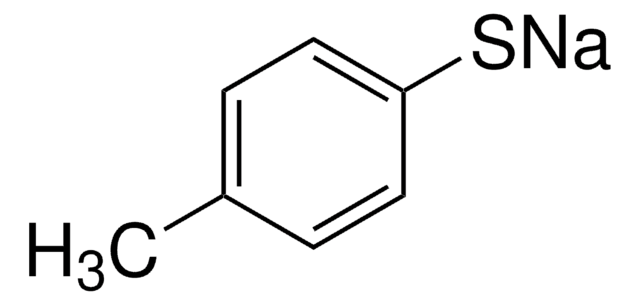
Sodium thiophenolate
technical grade, 90%
Synonym(s):
Benzenethiol sodium salt, Sodium thiophenoxide, Thiophenol sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5SNa
CAS Number:
Molecular Weight:
132.16
Beilstein:
3597302
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
powder
mp
>300 °C (lit.)
SMILES string
[Na]Sc1ccccc1
InChI
1S/C6H6S.Na/c7-6-4-2-1-3-5-6;/h1-5,7H;/q;+1/p-1
InChI key
RZWQDAUIUBVCDD-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Sodium thiophenolate can be prepared from the reaction of sodium and thiophenol in diethyl ether.
Application
Sodium thiophenolate has been used for the synthesis of MCoTI-I and MCoTI-II cyclotides, which are naturally-occurring cyclic cystine-knot microprotein trypsin inhibitors. It may be employed in the following studies:
- As probe for the immunoassay and for the detection of label-free protein by surface-enhanced Raman scattering (SERS).
- Preparation of new cyclometalated 6-phenyl-4-(p-R-phenyl)-2,2′-bipyridyl (C--N--N)Pt(II) thiophenolate complexes.
- Synthesis of 1,3,5,7,9-pentakis(4-methoxyphenylthio)corannulene, 1,3,5,7,9-pentakis(2-naphthylthio)corannulene and 1,3,6,8-tetrakis(4-methoxyphenylthio)corannulene.
- Synthesis of Et4N+ salts of homoleptic arylthiolate Ti(IV) complex, [Ti(SPh)6]2-.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xuefang Gu et al.
Analytical and bioanalytical chemistry, 406(7), 1885-1894 (2014-03-01)
A simple and sensitive method, based on surface-enhanced Raman scattering (SERS), for immunoassay and label-free protein detection is reported. A series of bowl-shaped silver cavity arrays were fabricated by electrodeposition using a self-assembled polystyrene spheres template. The reflection spectra of
The synthesis and structural characterization of [Et4N]2 [Ti(SPh)6] and trimeric [Ti3O(SPh)3Cl4 (CH3 CN)5]?CH3 CN?(C2 H5)2O complexes.
Kim JT, et al.
Polyhedron, 19(9), 1139-1143 (2000)
Jacob Schneider et al.
Inorganic chemistry, 48(4), 1498-1506 (2009-01-15)
Three new cyclometalated 6-phenyl-4-(p-R-phenyl)-2,2'-bipyridyl (C--N--N) Pt(II) thiophenolate complexes (R = Me (2a), COOMe (2b), and P(O)(OEt)(2) (2c)) have been synthesized and studied. The new C--N--N ligands L2 (R = COOMe) and L3 (R = P(O)(OEt)(2)) undergo cyclometalation with a Pt(II)
Panumart Thongyoo et al.
Organic & biomolecular chemistry, 6(8), 1462-1470 (2008-04-04)
The naturally-occurring cyclic cystine-knot microprotein trypsin inhibitors MCoTI-I and MCoTI-II have been synthesised using both thia-zip native chemical ligation and a biomimetic strategy featuring chemoenzymatic cyclisation by an immobilised protease. Engineered analogues have been produced containing a range of substitutions
S Mizyed et al.
Journal of the American Chemical Society, 123(51), 12770-12774 (2001-12-26)
1,3,5,7,9-Pentakis(4-methoxyphenylthio)corannulene (3), 1,3,5,7,9-pentakis(2-naphthylthio)corannulene (4), and 1,3,6,8-tetrakis(4-methoxyphenylthio)corannulene (5b) have been synthesized by chlorination of corannulene with ICl in CH(2)Cl(2) at 25 degrees C and subsequent nucleophilic aromatic substitution with the appropriate sodium thiophenolate in DMEU at 25 degrees C. (1)H NMR
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service