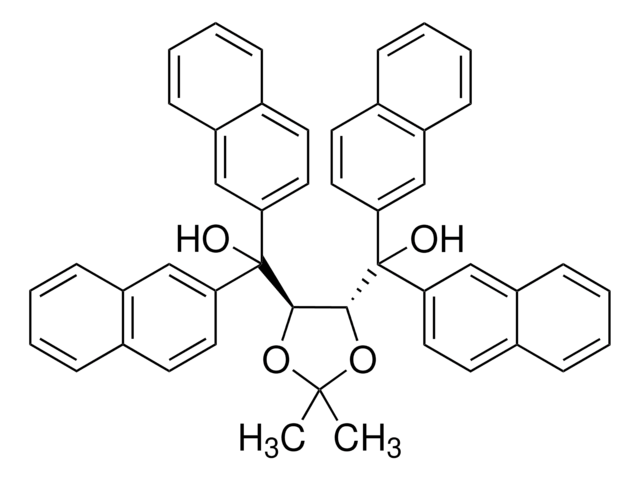
393762
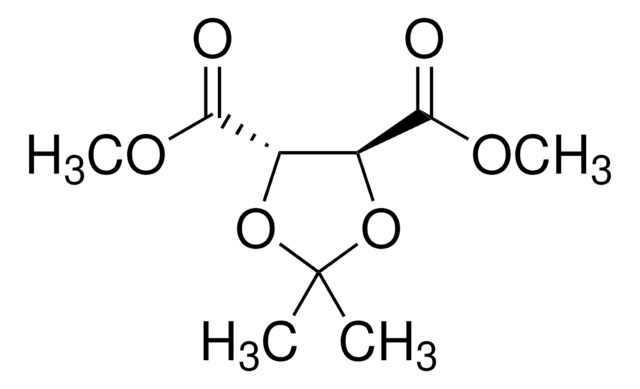
(4S-trans)-2,2-Dimethyl-α,α,α′,α′-tetra(2-naphthyl)-1,3-dioxolane-4,5-dimethanol
98%
Synonym(s):
(+)-2,3-O-Isopropylidene-1,1,4,4-tetra(2-naphthyl)-D-threitol, (+)-DINOL, (4S,5S)-2,2-Dimethyl-α,α,α′,α′-tetra(2-naphthyl)dioxolane-4,5-dimethanol
About This Item
Recommended Products
Assay
98%
optical activity
[α]20/D +116°, c = 1 in ethyl acetate
optical purity
ee: 99% (HPLC)
mp
214-216 °C (lit.)
SMILES string
CC1(C)O[C@@H]([C@H](O1)C(O)(c2ccc3ccccc3c2)c4ccc5ccccc5c4)C(O)(c6ccc7ccccc7c6)c8ccc9ccccc9c8
InChI
1S/C47H38O4/c1-45(2)50-43(46(48,39-23-19-31-11-3-7-15-35(31)27-39)40-24-20-32-12-4-8-16-36(32)28-40)44(51-45)47(49,41-25-21-33-13-5-9-17-37(33)29-41)42-26-22-34-14-6-10-18-38(34)30-42/h3-30,43-44,48-49H,1-2H3/t43-,44-/m0/s1
InChI key
QWADMRIAKWGVBF-CXNSMIOJSA-N
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The chiral auxiliaries TADDOLs (α,α,α,α-tetraaryl-1,3-dioxolane-4,5- dimethanols) developed by Seebach's group have found numerous applications in asymmetric synthesis ranging from utilization as stoichiometric chiral reagents or in Lewis acid mediated reactions, to roles in catalytic hydrogenation and stereoregular metathesis polymerization.
Apart from numerous examples using TADDOLs in metal-catalyzed asymmetric reactions, Rawal recently reported that TADDOLs could be used as Brønsted acid organocatalysts in highly stereoselective hetero-Diels–Alder reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Chlorocyclopentadienyl[(4R,5R)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanolato]titanium 97%](/deepweb/assets/sigmaaldrich/product/structures/232/672/2ca86719-0965-4619-b25b-aae4087a2aad/640/2ca86719-0965-4619-b25b-aae4087a2aad.png)