393193
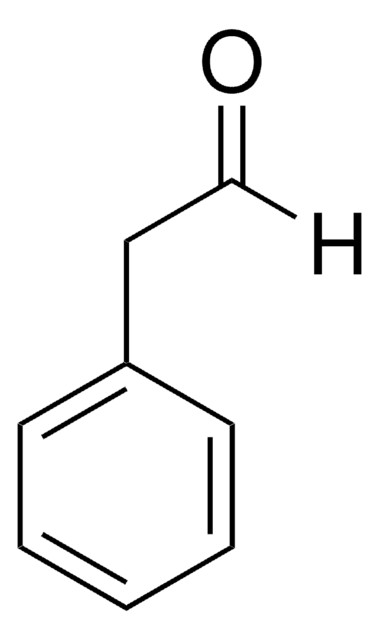
Hydrocinnamaldehyde
technical grade, 90%
Synonym(s):
3-Phenylpropionaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2CH2CHO
CAS Number:
Molecular Weight:
134.18
Beilstein:
1071910
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
liquid
impurities
<5% 3-phenyl-1-propanol
refractive index
n20/D 1.523 (lit.)
bp
97-98 °C/12 mmHg (lit.)
density
1.019 g/mL at 25 °C (lit.)
functional group
aldehyde
phenyl
SMILES string
[H]C(=O)CCc1ccccc1
InChI
1S/C9H10O/c10-8-4-7-9-5-2-1-3-6-9/h1-3,5-6,8H,4,7H2
InChI key
YGCZTXZTJXYWCO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hydrocinnamaldehyde is C=C double bond hydrogenated cinnamaldehyde. It has been synthesized by the selective hydrogenation of C=C double bond of cinnamaldehyde by various methods. Hydrocinnamaldehyde and nitromethane undergoes Henry reaction to form nitroaldols. The C1 and C3 position labelled with 13C of hydrocinnamaldehyde was subjected to mass spectrometry and the fragmentation pattern was elucidated.
Application
Hydrocinnamaldehyde may be used in the synthesis of the following:
- 2-Chloro hydrocinnamaldehyde by α-chlorination.
- Mixture of homopropargyl alcohols by kinetic resolution-allenylboration reactions.
- Mixture of syn- and anti-β-hydroxyallylsilanes by hydroboration of allenylsilane.
- (2S)-2-Hydroxy-4-phenylbutanenitrile by catalytic asymmetric cyanosilylation.
- Cinnamaldehyde by dehydrogenation reaction using Pd(TFA)2/4,5-diazafluorenone catalyst.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
203.0 °F - closed cup
Flash Point(C)
95 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Metal versus silyl triflate catalysis in the Mukaiyama aldol addition reaction.
Carreira EM and Singer RA
Tetrahedron Letters, 35(25), 4323-4326 (1994)
Elucidation of elctron-impact-induced fragmentations of hydrocinnamaldehyde by 13C labelling; evidence for an 'ortho attack'in the molecular ion.
Venema A and Nibbering NMM
Org. Mass Spectrom., 9(6), 628-630 (1974)
Carbon nanofiber supported palladium catalyst for liquid-phase reactions: An active and selective catalyst for hydrogenation of cinnamaldehyde into hydrocinnamaldehyde.
Pham-Huu C, et al
J. Mol. Catal. A: Chem., 170(1), 155-163 (2001)
Ming Chen et al.
Organic letters, 13(8), 1992-1995 (2011-03-18)
The kinetic hydroboration of allenylsilane 5 with ((d)Ipc)(2)BH at -40 °C provides allylborane 9Z with ≥12:1 selectivity. When the hydroboration is performed at temperatures above -40 °C, 9Z isomerizes to the thermodynamically more stable allylborane 9E with >20:1 selectivity. Subsequent
Influence of Preparation Modes on Pt-Ni/CNTs Catalysts Used in the Selective Hydrogenation of Cinnamaldehyde to Hydrocinnamaldehyde.
Li Y, et al
Catalysis Letters, 126(3-4), 280-285 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service