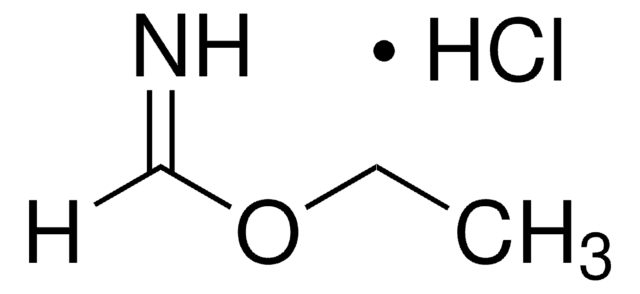
391204
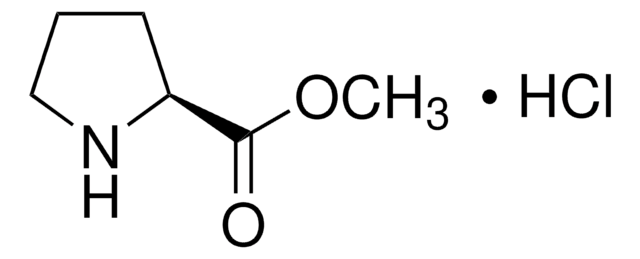
Methyl pipecolinate hydrochloride
97%
Synonym(s):
Methyl 2-piperidinecarboxylate hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H13NO2 · HCl
CAS Number:
Molecular Weight:
179.64
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
205 °C (dec.) (lit.)
functional group
ester
SMILES string
Cl.COC(=O)C1CCCCN1
InChI
1S/C7H13NO2.ClH/c1-10-7(9)6-4-2-3-5-8-6;/h6,8H,2-5H2,1H3;1H
InChI key
APCHKWZTSCBBJX-UHFFFAOYSA-N
General description
Methyl pipecolinate hydrochloride is a hydrochloride salt of methyl piperidine-2-carboxylate (methyl pipecolinate). The kinetics of the enzymatic separation of enantiomeric forms of methyl pipecolinate using Candida antarctica Lipase A (CAL-A) has been reported. Its role as catalyst for the standard Diels-Alder reaction has been examined.
Application
Reactant for synthesis of:
A pipecolic linker
Antiviral agents
Aurora and epidermal growth factor receptor kinase inhibitor
Pyrrolidine derivatives via reduction of substituted pyrroles
Reactant for:
Petasis reactions
Decarbonylative radical cyclization of alpha-amino selenoesters upon electrophilic alkenes
A pipecolic linker
Antiviral agents
Aurora and epidermal growth factor receptor kinase inhibitor
Pyrrolidine derivatives via reduction of substituted pyrroles
Reactant for:
Petasis reactions
Decarbonylative radical cyclization of alpha-amino selenoesters upon electrophilic alkenes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Advances in the kinetic and dynamic kinetic resolution of piperazine-2-carboxylic acid derivatives with Candida antarctica lipase A; structural requirements for enantioselective N-acylation.
Hietanen A, et al.
ARKIVOC (Gainesville, FL, United States), 5, 60-74 (2012)
Aldehyde-based racemization in the dynamic kinetic resolution of N-heterocyclic a-amino esters using Candida Antarctica lipase A.
Liljeblad A, et al.
Tetrahedron, 60(3), 671-677 (2004)
The α-effect in cyclic secondary amines: new scaffolds for iminium ion accelerated transformations.
Brazier JB, et al.
Tetrahedron, 65(48), 9961-9966 (2009)
Enantioselective lipase-catalyzed reactions of methyl pipecolinate: transesterification and N-acylation.
Liljeblad, A, et al.
Tetrahedron Letters, 43(13), 2471-2474 (2002)
Alkoxycarbonylpiperidines as N-nucleophiles in the palladium-catalyzed aminocarbonylation.
Takacs A, et al.
Monatshefte fur Chemie / Chemical Monthly, 145(9), 1473-1478 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service