275603
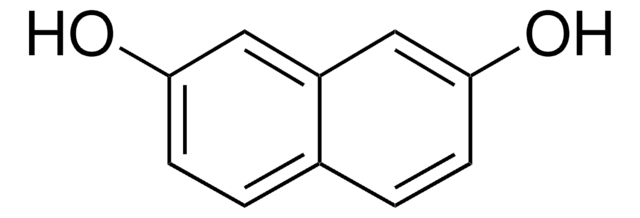
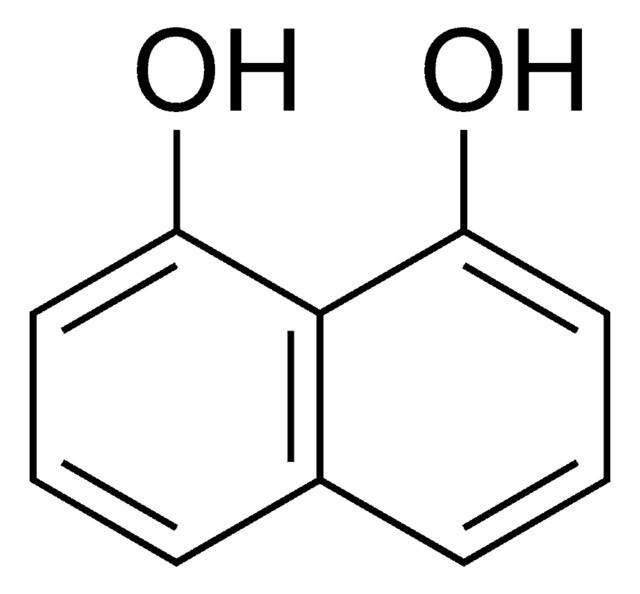
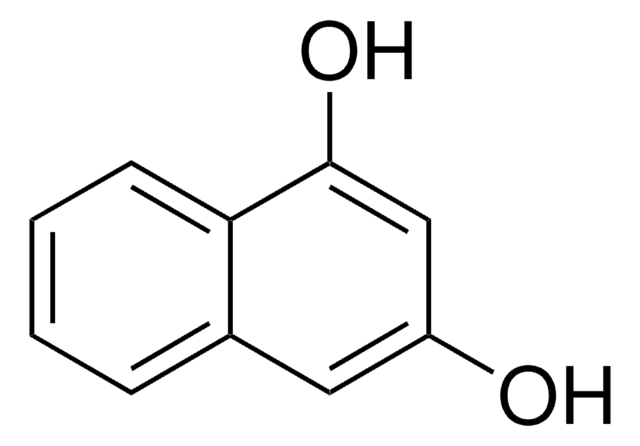
2,6-Dihydroxynaphthalene
98%
Synonym(s):
2,6-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H6(OH)2
CAS Number:
Molecular Weight:
160.17
Beilstein:
1238082
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
223-225 °C (lit.)
SMILES string
Oc1ccc2cc(O)ccc2c1
InChI
1S/C10H8O2/c11-9-3-1-7-5-10(12)4-2-8(7)6-9/h1-6,11-12H
InChI key
MNZMMCVIXORAQL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,6-Dihydroxynaphthalene was used in the synthesis of 1,5-dichloro-2,6-diethynylnaphthalenes. It was also used in the preparation of first-generation rotaxane dendrimer.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shoji Shinamura et al.
The Journal of organic chemistry, 75(4), 1228-1234 (2010-01-27)
In this paper we present the synthesis, structures, characterization, and applications to field-effect transistors (FETs) of naphtho[1,2-b:5,6-b']dithiophene (NDT) and -diselenophene (NDS) derivatives. Treatment of 1,5-dichloro-2,6-diethynylnaphthalenes, easily derived from commercially available 2,6-dihydroxynaphthalene, with sodium chalcogenide afforded a straightforward access to NDTs
Soo-Young Kim et al.
Chemistry, an Asian journal, 2(6), 747-754 (2007-05-08)
By taking advantage of the fact that cucurbit[6]uril (CB[6]) forms exceptionally stable host-guest complexes with protonated amines, and that its homologue CB[8] can encapsulate a pair of electron-rich and electron-deficient guest molecules to form a stable 1:1:1 complex, we synthesized
Alberto Macone et al.
Bioorganic & medicinal chemistry, 17(16), 6003-6007 (2009-07-21)
Aromatic substrates tyrosol (p-hydroxyphenylethanol) and 2,6-dihydroxynaphthalene (2,6-DHN) were converted into chromane derivatives by means of chemoenzymatic reactions catalyzed by the aromatic prenyltransferase of bacterial origin NovQ, using dimethylallyl bromide as allylic substrate instead of the natural isoprenyl pyrophosphate substrate. Stereoselective
Mårten Jacobsson et al.
Journal of medicinal chemistry, 49(6), 1932-1938 (2006-03-17)
The antiproliferative activity of the 14 isomeric monoxylosylated dihydroxynaphthalenes has been tested in vitro toward normal HFL-1 and 3T3 A31 cells as well as transformed T24 and 3T3 SV40 cells. The antiproliferative effect toward HFL-1 cells was correlated with the
Dieter Schemeth et al.
Analytica chimica acta, 1038, 182-190 (2018-10-04)
In this study, we focus on isolation and fractionation strategies by solid phase extraction (SPE) for a broad range of environmentally related organic acids. These emerging potential contaminants are primary degradation products of spilled petrogenic compounds but little attention has
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service