270016
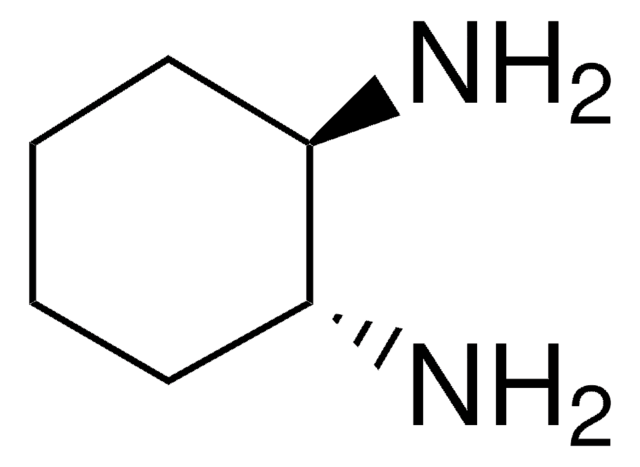
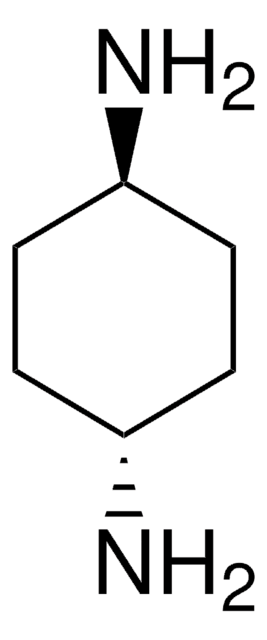
(±)-trans-1,2-Diaminocyclohexane
99%
Synonym(s):
(±)-trans-1,2-Cyclohexanediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H10(NH2)2
CAS Number:
Molecular Weight:
114.19
Beilstein:
3193807
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.4 mmHg ( 20 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.489 (lit.)
bp
79-81 °C/15 mmHg (lit.)
mp
14-15 °C (lit.)
density
0.951 g/mL at 25 °C (lit.)
SMILES string
N[C@@H]1CCCC[C@H]1N
InChI
1S/C6H14N2/c7-5-3-1-2-4-6(5)8/h5-6H,1-4,7-8H2/t5-,6-/m1/s1
InChI key
SSJXIUAHEKJCMH-PHDIDXHHSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(±)-trans-1,2-Diaminocyclohexane is widely used as a ligand in coordination chemistry and organocatalysis. It also serves as an achiral ligand in asymmetric catalysis. (±)-trans-1,2-Diaminocyclohexane was condensed with aliphatic dialdehydes to form [3+3] or [2+2] macrocyclization products.
Application
(±)-trans-1,2-Diaminocyclohexane was used in the synthesis of macrocyclic [3+3] hexa Schiff base. It was also employed in the synthesis of multidentate ligands, chiral auxiliaries and chiral stationary phases.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chirality, 4, 447-447 (1992)
Tetrahedron, 49, 4419-4419 (1993)
Jana Hodacová et al.
Organic letters, 9(26), 5641-5643 (2007-11-22)
2,2'-Bipyridine-5,5'-dicarbaldehyde has been prepared in two steps by enamination of 5,5'-dimethyl-2,2'-bipyridine with Bredereck's reagent, and subsequent oxidative cleavage of the enamine groups with sodium periodate. On condensation of this dialdehyde with enantiomerically pure trans-1,2-diaminocyclohexane, the macrocyclic [3+3] hexa Schiff base
Marcin Kwit et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(31), 8688-8695 (2007-07-31)
Aliphatic dialdehydes of rigid structures having a cyclohexane, a bicyclo[2.2.2]octane or a [7]triangulane skeleton, have been condensed with enantiomerically pure trans-1,2-diaminocyclohexane to give [3+3] or [2+2] macrocyclization products. Unlike acyclic aliphatic imines, these macrocyclic oligoimines show enhanced stabilities and their
The Journal of Organic Chemistry, 56, 3339-3339 (1991)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 270016-10ML | 4061826141717 |
| 270016-250ML | 4061826141724 |
| 270016-50ML | 4061826141731 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service