All Photos(1)
About This Item
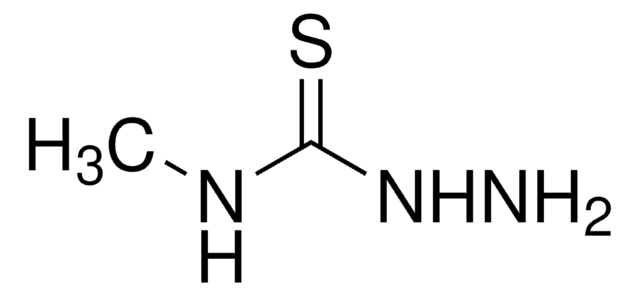
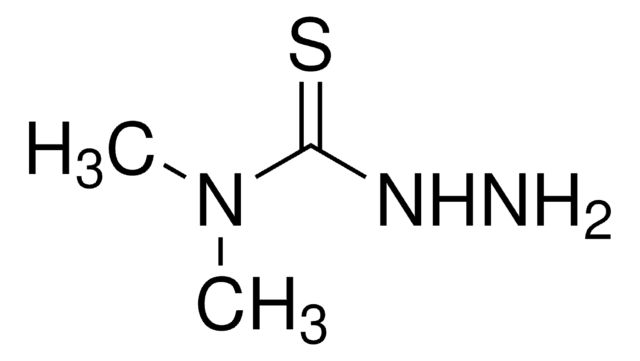
Linear Formula:
H2NCSN(CH3)NH2
CAS Number:
Molecular Weight:
105.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
173-175 °C (lit.)
SMILES string
CN(N)C(N)=S
InChI
1S/C2H7N3S/c1-5(4)2(3)6/h4H2,1H3,(H2,3,6)
InChI key
IIGQLQZSWDUOBI-UHFFFAOYSA-N
Related Categories
General description
The rate constant for dihydroxy intermediate formation from 2-methyl-3-thiosemicarbazide was studied.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reaction between various benzaldehydes and phenylhydroxylamine: special behaviour compared with other amines.
Brighente IM, et al.
J. Chem. Soc. Perkin Trans. II, 11, 1861-1864 (1991)
Synthesis and characterization of new amino acyl-4-thiazolidones.
Leite ACL, et al.
Quimica nova, 30(2), 284-286 (2007)
Franco Bisceglie et al.
Metallomics : integrated biometal science, 8(12), 1255-1265 (2016-11-15)
A comparative study between two bisthiosemicarbazones, 2,3-butanedione bis(4,4-dimethyl-3-thiosemicarbazone) and 2,3-butanedione bis(2-methyl-3-thiosemicarbazone), and their copper(ii) complexes is reported. The four compounds have been tested on a leukemia cell line U937 (p53-null) and on an adenocarcinoma cell line A549. The study includes
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 264989-1G | 4061826198100 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service