255750
Zinc oxide
99.99% trace metals basis
Synonym(s):
Zinc monoxide, Zinc white
About This Item
Recommended Products
Assay
99.99% trace metals basis
form
powder
SMILES string
O=[Zn]
InChI
1S/O.Zn
InChI key
XLOMVQKBTHCTTD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Schottky diodes that are applied in radio-frequency energy-harvesting circuits.
- ZnO nanostructures for sensing applications. For example, it can be used to fabricate Cr2O3-ZnCr2O4 hetero-nanostructures for highly selective xylene sensors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
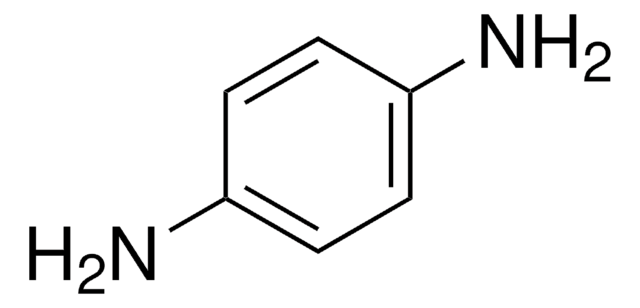
Conducting polymers such as polyaniline, polythiophene and polyfluorenes are now much in the spotlight for their applications in organic electronics and optoelectronics.
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service