253030
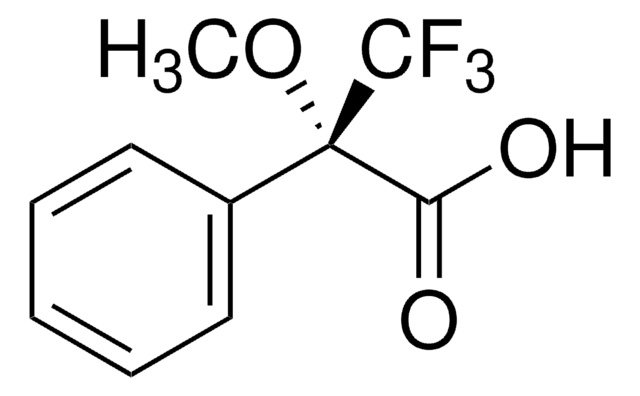
(R)-(−)-O-Acetylmandelic acid
99%, optical purity ee: 98% (GLC)
Synonym(s):
(−)-O-Acetyl-D-mandelic acid, (R)-(−)-α-Acetoxyphenylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
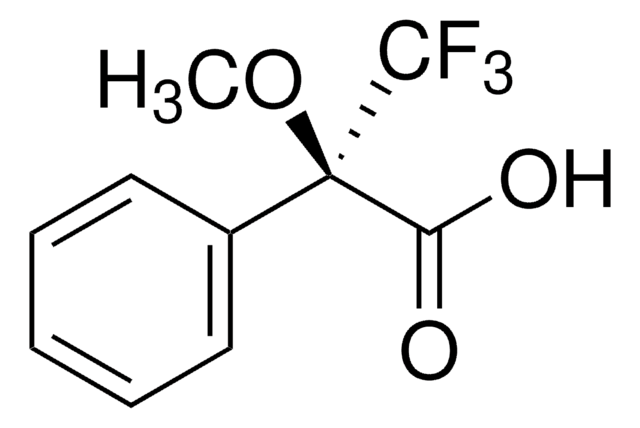
CH3CO2CH(C6H5)CO2H
CAS Number:
Molecular Weight:
194.18
Beilstein:
2694110
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
optical activity
[α]20/D −152.4°, c = 2 in acetone
optical purity
ee: 98% (GLC)
mp
97-99 °C (lit.)
functional group
carboxylic acid
ester
phenyl
SMILES string
CC(=O)O[C@@H](C(O)=O)c1ccccc1
InChI
1S/C10H10O4/c1-7(11)14-9(10(12)13)8-5-3-2-4-6-8/h2-6,9H,1H3,(H,12,13)/t9-/m1/s1
InChI key
OBCUSTCTKLTMBX-SECBINFHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(R)-(-)-O-Acetylmandelic acid is a chiral derivatizing agent for NMR determination of enantiomeric purity of α-deuterated carboxylic acids, alcohols, and amines.
Application
(R)-(-)-O-Acetylmandelic acid may be used as a precursor to prepare a chiral diamine, which is an intermediate to prepare Utenone A. It may also be used to prepare ethyl (2′R)-2′-acetoxy-2′-phenylethanoate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tritiated chiral alkanes as substrates for soluble methane monooxygenase from Methylococcus capsulatus (Bath): probes for the mechanism of hydroxylation.
Valentine AM, et al.
Journal of the American Chemical Society, 119(8), 1818-1827 (1997)
Chiroptical features and luminescence behaviour of macrocyclic tetra (4-quinolyl)-complexes: surprising absence of exciton coupling.
Govenlock L, et al.
J. Chem. Soc. Perkin Trans. II, 11, 2415-2418 (1999)
Modular ligand variation in calcium bisimidazoline complexes: effects on ligand redistribution and hydroamination catalysis.
Wixey JS and Ward BD.
Dalton Transactions, 40(30), 7693-7696 (2011)
An efficient synthesis of chiral nonracemic diamines: application in asymmetric synthesis.
Saravanan P and Singh VK.
Tetrahedron Letters, 39(1), 167-170 (1998)
J. Chem. Soc. Perkin Trans. II, 83-83 (1983)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service