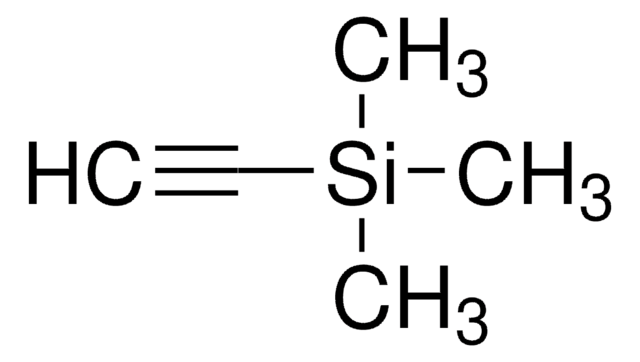244481
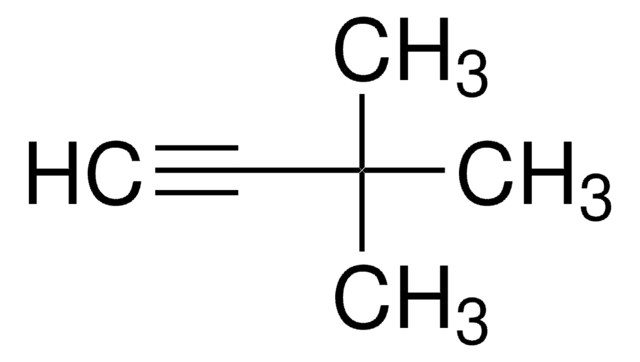
1-(Trimethylsilyl)propyne
99%
Synonym(s):
Trimethyl-1-propynylsilane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C≡CSi(CH3)3
CAS Number:
Molecular Weight:
112.24
Beilstein:
1071311
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.417 (lit.)
bp
99-100 °C (lit.)
density
0.758 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC#C[Si](C)(C)C
InChI
1S/C6H12Si/c1-5-6-7(2,3)4/h1-4H3
InChI key
DCGLONGLPGISNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-(Trimethylsilyl)propyne was used in the synthesis of highly substituted indenes via palladium-catalyzed carboannulation and indenones via a rhodium-catalyzed reaction with 2-bromophenylboronic acids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
39.2 °F - closed cup
Flash Point(C)
4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daohua Zhang et al.
The Journal of organic chemistry, 72(1), 251-262 (2006-12-30)
The synthesis of highly substituted indenes has been achieved by three different transition metal-mediated methods. The first method involves the palladium-catalyzed carboannulation of internal alkynes. The second method utilizes a two-step approach, which involves first the palladium/copper-catalyzed cross-coupling of terminal
Tetrahedron Letters, 33, 5969-5969 (1992)
Yasuyuki Harada et al.
Journal of the American Chemical Society, 129(17), 5766-5771 (2007-04-10)
The Rh-catalyzed reaction of alkynes with 2-bromophenylboronic acids involves carbonylative cyclization to give indenones. The key steps in the reaction involve the addition of an arylrhodium(I) species to an alkyne and the oxidative addition of C-Br bonds on the adjacent
Journal of Polymer Science Part A: Polymer Chemistry, 25, 1353-1353 (1987)
Journal of the American Chemical Society, 105, 7473-7473 (1983)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service