About This Item
Recommended Products
Quality Level
Assay
98%
bp
227-228 °C (lit.)
mp
42-44 °C (lit.)
functional group
chloro
fluoro
SMILES string
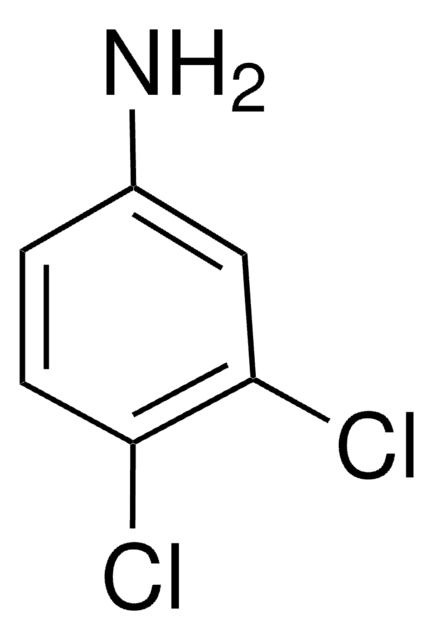
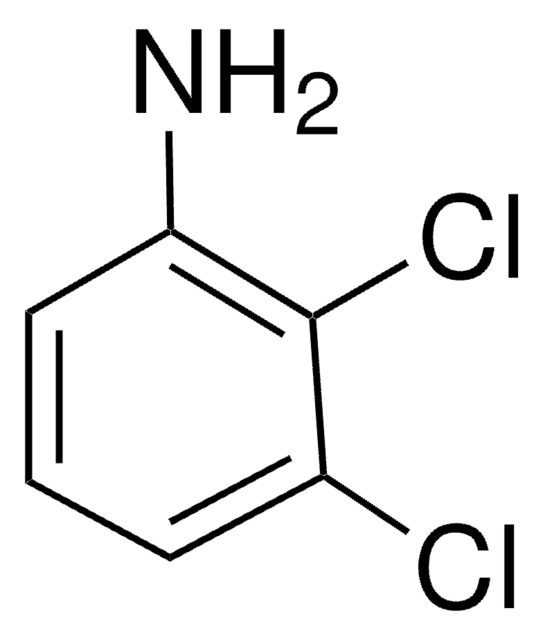
Nc1ccc(F)c(Cl)c1
InChI
1S/C6H5ClFN/c7-5-3-4(9)1-2-6(5)8/h1-3H,9H2
InChI key
YSEMCVGMNUUNRK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
Protocol for GC Analysis of Anilines on Equity®-5
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service