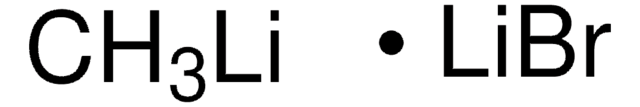197343
Methyllithium solution
1.6 M in diethyl ether
Synonym(s):
Lithium methanide, MeLi
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
CH3Li
CAS Number:
Molecular Weight:
21.98
Beilstein:
3587162
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3 (vs air)
Quality Level
form
liquid
composition
halide, ~0.05 M
concentration
1.6 M in diethyl ether
density
0.732 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
[Li]C
InChI
1S/CH3.Li/h1H3;
InChI key
DVSDBMFJEQPWNO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyllithium is an organolithium reagent mainly used for deprotonation and as a source of methyl anion. It is a strong base and a strong nucleophile.
Application
Methyllithium solution (1.6M in diethyl ether) has been used for the asymmetric allylic alkylation (AAA) of allylic electrophiles in the presence of a chiral copper catalyst. This protocol leads to the C-C bond formation of tertiary and quaternary stereogenic centers with high enantioselectivity. It can also be used for the synthesis of (−)-salsolidine by reacting with 6,7-dimethoxy-3,4-dihydroisoquinoline in the presence of (−)-sparteine as a chiral ligand.
Packaging
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
Legal Information
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kiran Kumar Solingapuram Sai et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 129, 57-61 (2017-08-15)
We automated radiochemical synthesis of 1-[
Enantioselective addition of organolithium reagents to 3, 4-dihydroisoquinoline in the presence of (?)-sparteine as an external ligand. Application for the synthesis of isoquinoline alkaloids.
Chrzanowska M
Tetrahedron Asymmetry, 12(10), 1435-1440 (2001)
A Simplified Approach with 3D Visuals
Vasishta Bhatt
Essentials of Coordination Chemistry: A Simplified Approach with 3D Visuals, 183-183 (2015)
Mingyang Zhang et al.
Macromolecular rapid communications, 41(18), e2000323-e2000323 (2020-08-11)
Carbon monoxide (CO) has emerged as a potential therapeutic agent for the treatment of many diseases. However, the therapeutic outcome is highly dependent on the dosages and administration sites. Hence, there is mounting interest in the development of CO-releasing materials
Cu-catalyzed enantioselective allylic alkylation with organolithium reagents.
Hornillos V, et al.
Nature Protocols, 12(3), 493-493 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service