All Photos(2)
About This Item
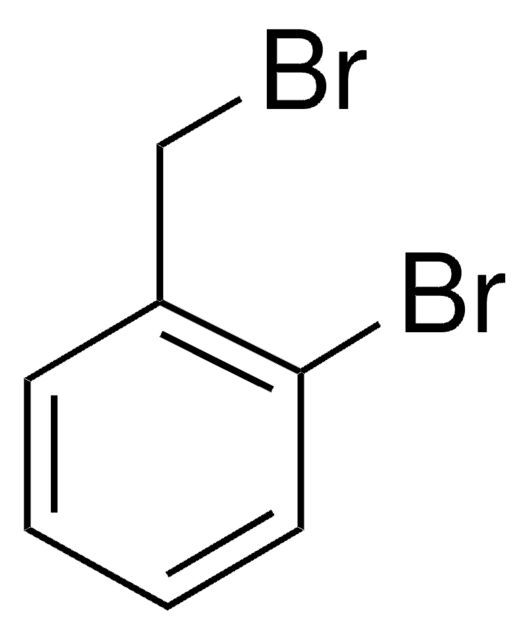
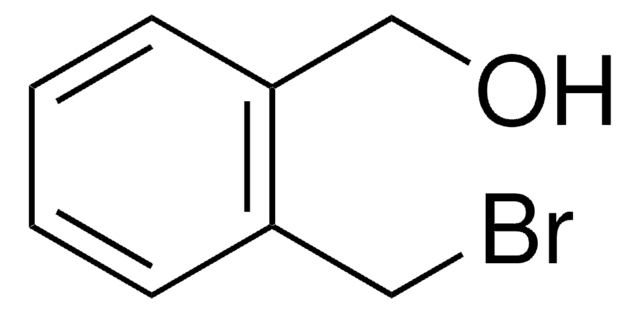
Linear Formula:
BrC6H4CH2OH
CAS Number:
Molecular Weight:
187.03
Beilstein:
2243418
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
78-80 °C (lit.)
functional group
bromo
hydroxyl
SMILES string
OCc1ccccc1Br
InChI
1S/C7H7BrO/c8-7-4-2-1-3-6(7)5-9/h1-4,9H,5H2
InChI key
IOWGHQGLUMEZKG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Bromobenzyl alcohol was used in microwave assisted palladium-catalyzed synthesis of phthalides. It was also used in the synthesis of 1-hydroxy-3(1H)-l,2-benzoboroxole.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and structure of benzoboroxoles: novel organoboron heterocycles.
Zhdankin VV, et al.
Tetrahedron Letters, 40(37), 6705-6708 (1999)
Fast microwave promoted palladium-catalyzed synthesis of phthalides from bromobenzyl alcohols utilizing DMF and Mo (CO)6 as carbon monoxide sources.
Wu X, et al.
Tetrahedron Letters, 45(24), 4635-4638 (2004)
Manas K Ghorai et al.
The Journal of organic chemistry, 79(14), 6468-6479 (2014-06-24)
A simple and unpredicted synthetic pathway toward racemic and scalemic tetrahydrodibenzoimidazoazepines has been invented serendipitously proceeding through an S(N)2-type ring-opening of N-activated aziridines with 2-bromobenzylamine followed by a hitherto unprecedented cascade cyclization reaction sequence comprising a Cu-catalyzed cross dehydrogenation C-N
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service