171565
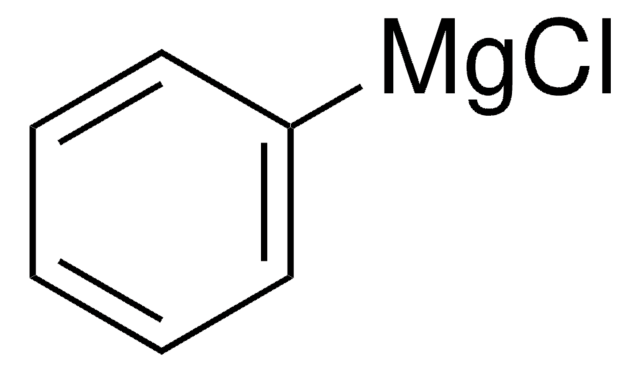
Phenylmagnesium bromide solution
3.0 M in diethyl ether
Synonym(s):
Bromomagnesiobenzene, Bromophenylmagnesium
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
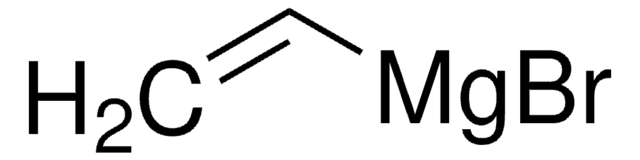
C6H5MgBr
CAS Number:
Molecular Weight:
181.31
Beilstein:
3588849
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
3.0 M in diethyl ether
density
1.134 g/mL at 25 °C
SMILES string
Br[Mg]c1ccccc1
InChI
1S/C6H5.BrH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
InChI key
ANRQGKOBLBYXFM-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Phenylmagnesium bromide solution contains 3M phenylmagnesium bromide in diethyl ether. It can act as a strong acid and Lewis acid. It can undergo addition reaction with many unsaturated functional groups. The phenyl group can displace halide from other organic compounds. Phenylmagnesium bromide is a Grignard reagent. Reaction of β-cyclohexanedione (dihydroresorcinol) with phenylmagnesium bromide has been investigated.
Application
Phenylmagnesium bromide was used for the synthesis of end-functionalized regioregular poly(3-alkylthiophene)s. It was also used for the monoalkylation of aliphatic primary amine to generate secondary amines by the Grignard reaction of 1-[(alkylamino) methyl] benzotriazoles.
It may be used for synthesis of the following:
It may be used for synthesis of the following:
- 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol
- 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol
- (3-(2-Dithiobenzoatepropionyl)propyl)dimethylmethoxysilane, reversible addition-fragmentation chain transfer polymerization (RAFT)-silane agent
- series of o-substituted benzophenones
Packaging
Other Notes
Storage below 25°C may cause formation of crystalline magnesium salts. Moving container to a warm location and occasional gentle swirling should redissolve the solid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-40.0 °F - closed cup
Flash Point(C)
-40 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of O-substituted benzophenones by Grignard reaction of 3-substituted isocoumarins.
Manivel P, et al.
Journal of the Chilean Chemical Society, 53(3), 1609-1610 (2008)
The reaction of beta-cyclohexanedione (dihydroresorcinol) and its ethyl enol ether with phenylmagnesium bromide.
G F WOODS et al.
Journal of the American Chemical Society, 70(6), 2174-2177 (1948-06-01)
Synthesis of well-defined polymer brushes grafted onto silica nanoparticles via surface reversible addition-fragmentation chain transfer polymerization.
Li C and Benicewicz BC.
Macromolecules, 38(14), 5929-5936 (2005)
S Schenone et al.
Farmaco (Societa chimica italiana : 1989), 45(12), 1309-1325 (1990-12-01)
The synthesis of 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol 2 and 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol 3 starting from (+)-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-one and phenylmagnesium bromide or benzylmagnesium chloride, respectively, is described. Alcohols 2 and 3 gave a series of omega-dialkylaminoalkyl ethers 4 by reaction as sodium salts with omega-chloroalkyldialkylamines in toluene
Phenylmagnesium Bromide.
Richey HG, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2009)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 171565-100ML | 4061838751058 |
| 171565-18L | 4061838751065 |
| 171565-800ML | 4061838751089 |
| 171565-50ML | 4061838751072 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service